- Record: found
- Abstract: found
- Article: found
Antibodies to a Citrullinated Porphyromonas gingivalis Epitope Are Increased in Early Rheumatoid Arthritis, and Can Be Produced by Gingival Tissue B Cells: Implications for a Bacterial Origin in RA Etiology
Read this article at
Abstract
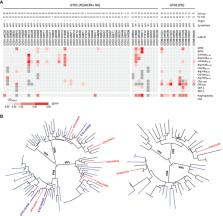
Based on the epidemiological link between periodontitis and rheumatoid arthritis (RA), and the unique feature of the periodontal bacterium Porphyromonas gingivalis to citrullinate proteins, it has been suggested that production of anti-citrullinated protein antibodies (ACPA), which are present in a majority of RA patients, may be triggered in the gum mucosa. To address this hypothesis, we investigated the antibody response to a citrullinated P. gingivalis peptide in relation to the autoimmune ACPA response in early RA, and examined citrulline-reactivity in monoclonal antibodies derived from human gingival B cells. Antibodies to a citrullinated peptide derived from P. gingivalis (denoted CPP3) and human citrullinated peptides were analyzed by multiplex array in 2,807 RA patients and 372 controls; associations with RA risk factors and clinical features were examined. B cells from inflamed gingival tissue were single-cell sorted, and immunoglobulin (Ig) genes were amplified, sequenced, cloned and expressed (n=63) as recombinant monoclonal antibodies, and assayed for citrulline-reactivities by enzyme-linked immunosorbent assay. Additionally, affinity-purified polyclonal anti-cyclic-citrullinated peptide (CCP2) IgG, and monoclonal antibodies derived from RA blood and synovial fluid B cells (n=175), were screened for CPP3-reactivity. Elevated anti-CPP3 antibody levels were detected in RA (11%), mainly CCP2+ RA, compared to controls (2%), p<0.0001, with a significant association to HLA-DRB1 shared epitope alleles, smoking and baseline pain, but with low correlation to autoimmune ACPA fine-specificities. Monoclonal antibodies derived from gingival B cells showed cross-reactivity between P. gingivalis CPP3 and human citrullinated peptides, and a CPP3+/CCP2+ clone, derived from an RA blood memory B cell, was identified. Our data support the possibility that immunity to P. gingivalis derived citrullinated antigens, triggered in the inflamed gum mucosa, may contribute to the presence of ACPA in RA patients, through mechanisms of molecular mimicry.
Related collections
Most cited references60

- Record: found
- Abstract: found
- Article: found
Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line
- Record: found
- Abstract: found
- Article: not found
Long-Lived Plasma Cells Are Contained within the CD19(-)CD38(hi)CD138(+) Subset in Human Bone Marrow.
- Record: found
- Abstract: found
- Article: not found