- Record: found
- Abstract: found
- Article: found
Inter- and intratumoral heterogeneity of BCL2 correlates with IgH expression and prognosis in follicular lymphoma
Read this article at
Abstract
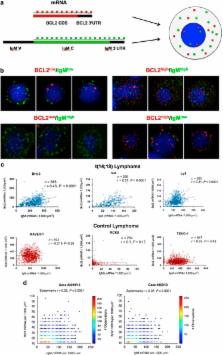
Most follicular lymphomas (FLs) are genetically defined by the t(14;18)(q32;q21) translocation that juxtaposes the BCL2 gene to the immunoglobulin heavy chain (IgH) 3' regulatory regions (IgH-3'RRs). Despite this recurrent translocation, FL cases are heterogeneous in terms of intratumoral clonal diversity for acquired mutations and variations in the tumor microenvironment. Here we describe an additional mechanism that contributes to inter- and intratumoral heterogeneity in FLs. By applying a novel single-molecule RNA fluorescence-based in situ hybridization (FISH) technique to detect mRNA molecules of BCL2 and IgH in single cells, we found marked heterogeneity in the number of BCL2 mRNA transcripts within individual lymphoma cells. Moreover, BCL2 mRNA molecules correlated with IgH mRNA molecules in individual cells both in t(14;18) lymphoma cell lines and in patient samples. Consistently, a strong correlation between BCL2 and IgH protein levels was found in a series of 205 primary FL cases by flow cytometry and immunohistochemistry. Inter- and intratumoral heterogeneity of BCL2 expression determined resistance to drugs commonly used in FL treatment and affected overall survival of FL patients. These data demonstrate that BCL2 and IgH expressions are heterogeneous and coregulated in t(14;18)-translocated cells, and determine the response to therapy in FL patients.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project.
- Record: found
- Abstract: found
- Article: not found
Involvement of the bcl-2 gene in human follicular lymphoma.
- Record: found
- Abstract: found
- Article: not found