- Record: found
- Abstract: found
- Article: found
Human Cytomegalovirus UL7, miR-US5-1, and miR-UL112-3p Inactivation of FOXO3a Protects CD34 + Hematopoietic Progenitor Cells from Apoptosis
Read this article at
Abstract
Human cytomegalovirus (HCMV) causes serious disease in immunocompromised individuals and is a significant problem during transplantation. The virus can establish a latent infection in CD34 + hematopoietic progenitor cells (HPCs) and periodically reactivate to cause disease in the absence of an intact immune system.
ABSTRACT
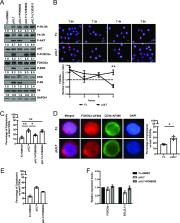
Human cytomegalovirus (HCMV) infection of myeloid lineage cells, such as CD34 + hematopoietic progenitor cells (HPCs) or monocytes, results in the upregulation of antiapoptotic cellular proteins that protect the newly infected cells from programmed cell death. The mechanisms used by HCMV to regulate proapoptotic cellular proteins upon infection of CD34 + HPCs have not been fully explored. Here, we show that HCMV utilizes pUL7, a secreted protein that signals through the FLT3 receptor, and miR-US5-1 and miR-UL112-3p to reduce the abundance and activity of the proapoptotic transcription factor FOXO3a at early times after infection of CD34 + HPCs. Regulation of FOXO3a by pUL7, miR-US5-1, and miR-UL112 results in reduced expression of the proapoptotic BCL2L11 transcript and protection of CD34 + HPCs from virus-induced apoptosis. These data highlight the importance of both viral proteins and microRNAs (miRNAs) in protecting CD34 + HPCs from apoptosis at early times postinfection, allowing for the establishment of latency and maintenance of viral genome-containing cells.
IMPORTANCE Human cytomegalovirus (HCMV) causes serious disease in immunocompromised individuals and is a significant problem during transplantation. The virus can establish a latent infection in CD34 + hematopoietic progenitor cells (HPCs) and periodically reactivate to cause disease in the absence of an intact immune system. What viral gene products are required for successful establishment of latency is still not fully understood. Here, we show that both a viral protein and viral miRNAs are required to prevent apoptosis after infection of CD34 + HPCs. HCMV pUL7 and miRNAs miR-US5-1 and miR-UL112-3p act to limit the expression and activation of the transcription factor FOXO3a, which in turn reduces expression of proapoptotic gene BCL2L11 and prevents virus-induced apoptosis in CD34 + HPCs.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor.
- Record: found
- Abstract: found
- Article: not found
FoxO transcription factors; Regulation by AKT and 14-3-3 proteins.
- Record: found
- Abstract: found
- Article: not found