- Record: found
- Abstract: found
- Article: found
Dysregulation of Ubiquitin-Proteasome System in Neurodegenerative Diseases
Read this article at
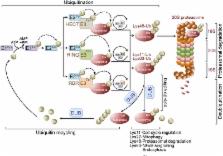
Abstract
The ubiquitin-proteasome system (UPS) is one of the major protein degradation pathways, where abnormal UPS function has been observed in cancer and neurological diseases. Many neurodegenerative diseases share a common pathological feature, namely intracellular ubiquitin-positive inclusions formed by aggregate-prone neurotoxic proteins. This suggests that dysfunction of the UPS in neurodegenerative diseases contributes to the accumulation of neurotoxic proteins and to instigate neurodegeneration. Here, we review recent findings describing various aspects of UPS dysregulation in neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: not found
Ubiquitination in disease pathogenesis and treatment.
- Record: found
- Abstract: found
- Article: not found
Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes.
- Record: found
- Abstract: found
- Article: not found