- Record: found
- Abstract: found
- Article: found
MAIT Cells in Barrier Tissues: Lessons from Immediate Neighbors
Read this article at
Abstract
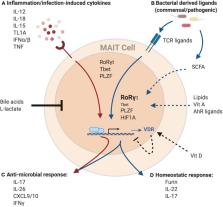
Mucosal-associated invariant T (MAIT) cells are innate-like T cells present at considerable frequencies in human blood and barrier tissues, armed with an expanding array of effector functions in response to homeostatic perturbations. Analogous to other barrier immune cells, their phenotype and function is driven by crosstalk with host and dynamic environmental factors, most pertinently the microbiome. Given their distribution, they must function in diverse extracellular milieus. Tissue-specific and adapted functions of barrier immune cells are shaped by transcriptional programs and regulated through a blend of local cellular, inflammatory, physiological, and metabolic mediators unique to each microenvironment. This review compares the phenotype and function of MAIT cells with other barrier immune cells, highlighting potential areas for future exploration. Appreciation of MAIT cell biology within tissues is crucial to understanding their niche in health and disease.
Related collections
Most cited references238
- Record: found
- Abstract: found
- Article: not found
Origin and physiological roles of inflammation.
- Record: found
- Abstract: found
- Article: not found
From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites.
- Record: found
- Abstract: found
- Article: not found