- Record: found
- Abstract: found
- Article: found
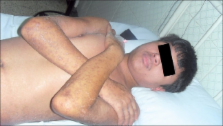
Oxcarbazepine-induced Stevens Johnson syndrome: A rare case report
case-report
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Although carbamazepine is the most common cause of Stevens Johnson syndrome (SJS) a new antiepileptic drug, oxcarbazepine which is structurally related to carbamazepine, has also been rarely shown to induce SJS. Here we report a case with SJS, which was induced by oxcarbazepine.
Related collections
Most cited references11
- Record: found
- Abstract: found
- Article: not found
Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel.
- Record: found
- Abstract: found
- Article: not found
Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study.
, Osvaldo Correia, Jean Claude Roujeau … (2002)
- Record: found
- Abstract: found
- Article: not found
Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics.
Pat Tennis, J Schlingmann, Maja Mockenhaupt … (2005)