- Record: found
- Abstract: found
- Article: found
Neurological Disease in Lupus: Toward a Personalized Medicine Approach
Read this article at
Abstract
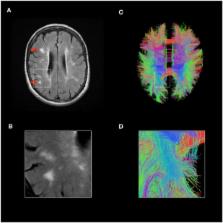
The brain and nervous system are important targets for immune-mediated damage in systemic lupus erythematosus (SLE), resulting in a complex spectrum of neurological syndromes. Defining nervous system disease in lupus poses significant challenges. Among the difficulties to be addressed are a diversity of clinical manifestations and a lack of understanding of their mechanistic basis. However, despite these challenges, progress has been made in the identification of pathways which contribute to neurological disease in SLE. Understanding the molecular pathogenesis of neurological disease in lupus will inform both classification and approaches to clinical trials.
Related collections
Most cited references118
- Record: found
- Abstract: not found
- Article: not found
Multiple sclerosis--the plaque and its pathogenesis.
- Record: found
- Abstract: found
- Article: not found