- Record: found
- Abstract: found
- Article: found
Willin/FRMD6 Influences Mechanical Phenotype and Neuronal Differentiation in Mammalian Cells by Regulating ERK1/2 Activity
Read this article at
Abstract
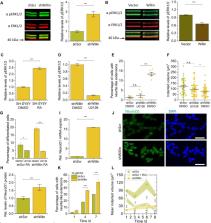
Willin/FRMD6 is part of a family of proteins with a 4.1 ezrin-radixin-moesin (FERM) domain. It has been identified as an upstream activator of the Hippo pathway and, when aberrant in its expression, is associated with human diseases and disorders. Even though Willin/FRMD6 was originally discovered in the rat sciatic nerve, most studies have focused on its functional roles in cells outside of the nervous system, where Willin/FRMD6 is involved in the formation of apical junctional cell-cell complexes and in regulating cell migration. Here, we investigate the biochemical and biophysical role of Willin/FRMD6 in neuronal cells, employing the commonly used SH-SY5Y neuronal model cell system and combining biochemical measurements with Elastic Resonator Interference Stress Micropscopy (ERISM). We present the first direct evidence that Willin/FRMD6 expression influences both the cell mechanical phenotype and neuronal differentiation. By investigating cells with increased and decreased Willin/FRMD6 expression levels, we show that Willin/FRMD6 not only affects proliferation and migration capacity of cells but also leads to changes in cell morphology and an enhanced formation of neurite-like membrane extensions. These changes were accompanied by alterations of biophysical parameters such as cell force, the organization of actin stress fibers and the formation of focal adhesions. At the biochemical level, changes in Willin/FRMD6 expression inversely affected the activity of the extracellular signal-regulated kinases (ERK) pathway and downstream transcriptional factor NeuroD1, which seems to prime SH-SY5Y cells for retinoic acid (RA)-induced neuronal differentiation.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Identification of a novel inhibitor of mitogen-activated protein kinase kinase.
- Record: found
- Abstract: found
- Article: not found
YAP/TAZ upstream signals and downstream responses
- Record: found
- Abstract: found
- Article: not found