- Record: found
- Abstract: found
- Article: found
Migrasome biogenesis and functions
Read this article at
Abstract
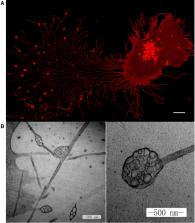
The migrasome is a newly discovered organelle produced by migrating cells. As cells migrate, long and thin retraction fibers are left in their wake. On these fibers, we discovered the production of a pomegranate‐like structure, which we named migrasomes. The production of migrasomes is highly correlated with the migration of cells. Currently, it has been demonstrated the migrasomes exhibit three modes of action: release of signaling molecules through rupturing or leaking, carriers of damaged mitochondria, and lateral transfer of mRNA or proteins. In this review, we would like to discuss, in detail, the functions, mechanisms, and potential applications of this newly discovered cell organelle.
Abstract
Migrasomes are newly found organelles produced by migrating cells and grow on retraction fibers trailing behind the cells. Migrasomes are produced as a result of tetraspanin‐enriched macrodomain accumulation and could be released into the extracellular matrix or engulfed by other cells. Migrasomes could act as carriers of mRNA, protein, or damaged mitochondria, or as chemoattractive sources.
Related collections
Most cited references18

- Record: found
- Abstract: found
- Article: found
Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration
- Record: found
- Abstract: found
- Article: not found
Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly
- Record: found
- Abstract: found
- Article: not found
