- Record: found
- Abstract: found
- Article: found
Solid phase synthesis and RNA-binding activity of an arginine-containing nucleopeptide†
Read this article at
Abstract
Abstract
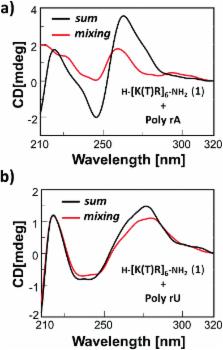
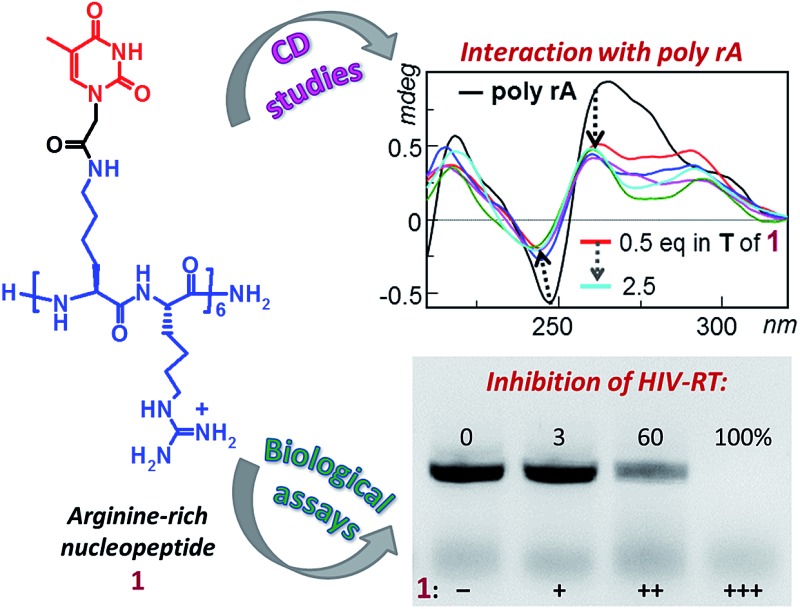
Here we report the solid phase synthesis and characterization (LC-ESIMS, CD) of a cationic nucleobase-containing α-peptide, composed of both l-arginine residues and l-lysine-based nucleoamino acids sequentially present in the structure. The binding properties of this novel basic nucleopeptide towards nucleic acids were investigated by CD spectroscopy which revealed the ability of the thymine-containing oligomer to bind both adenine-containing DNA (dA 12) and RNA (poly rA) molecules inducing high conformational variations in the nucleic acid structures. Moreover, the artificial oligonucleotide inhibited the enzymatic activity of HIV reverse transcriptase, opening the door to the exploitation of novel antiviral strategies inspired to this molecular tool.
Related collections
Most cited references36

- Record: found
- Abstract: found
- Article: found
Serum Stabilities of Short Tryptophan- and Arginine-Rich Antimicrobial Peptide Analogs
- Record: found
- Abstract: found
- Article: not found
Identification and functional characterization of neo-poly(A) polymerase, an RNA processing enzyme overexpressed in human tumors.
Author and article information
Notes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25809j