- Record: found
- Abstract: found
- Article: found
Taurodeoxycholate Increases the Number of Myeloid-Derived Suppressor Cells That Ameliorate Sepsis in Mice
Read this article at
Abstract
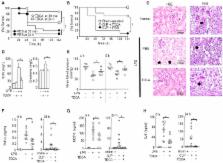
Bile acids (BAs) control metabolism and inflammation by interacting with several receptors. Here, we report that intravenous infusion of taurodeoxycholate (TDCA) decreases serum pro-inflammatory cytokines, normalizes hypotension, protects against renal injury, and prolongs mouse survival during sepsis. TDCA increases the number of granulocytic myeloid-derived suppressor cells (MDSC LT) distinctive from MDSCs obtained without TDCA treatment (MDSC L) in the spleen of septic mice. FACS-sorted MDSC LT cells suppress T-cell proliferation and confer protection against sepsis when adoptively transferred better than MDSC L. Proteogenomic analysis indicated that TDCA controls chromatin silencing, alternative splicing, and translation of the immune proteome of MDSC LT, which increases the expression of anti-inflammatory molecules such as oncostatin, lactoferrin and CD244. TDCA also decreases the expression of pro-inflammatory molecules such as neutrophil elastase. These findings suggest that TDCA globally edits the proteome to increase the number of MDSC LT cells and affect their immune-regulatory functions to resolve systemic inflammation during sepsis.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Identification of a nuclear receptor for bile acids.
- Record: found
- Abstract: found
- Article: not found
Purinergic regulation of the immune system.
- Record: found
- Abstract: found
- Article: not found