- Record: found
- Abstract: found
- Article: found
Modulation of Intestinal TLR4-Inflammatory Signaling Pathways by Probiotic Microorganisms: Lessons Learned from Lactobacillus jensenii TL2937
Read this article at
Abstract
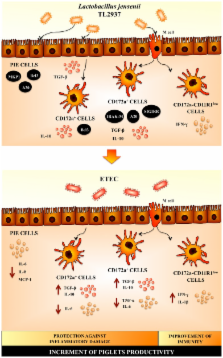
The intestinal mucosa plays a critical role in the host’s interactions with innocuous commensal microbiota and invading pathogenic microorganisms. Intestinal epithelial cells (IECs) and gut associated immune cells recognize the bacterial components via pattern-recognition receptors (PRRs) and are responsible for maintaining tolerance to the large communities of resident luminal bacteria while being also able to mount inflammatory responses against pathogens. Toll-like receptors (TLRs) are a major class of PRRs that are present on IECs and immune cells which are involved in the induction of both tolerance and inflammation. A growing body of experimental and clinical evidence supports the therapeutic and preventive application of probiotics for several gastrointestinal inflammatory disorders in which TLRs exert a significant role. This review aims to summarize the current knowledge of the beneficial effects of probiotic microorganisms with the capacity to modulate the immune system (immunobiotics) in the regulation of intestinal inflammation in pigs, which are very important as both livestock and human model. Especially we discuss the role of TLRs, their signaling pathways, and their negative regulators in both the inflammatory intestinal injury and the beneficial effects of immunobiotics in general, and Lactobacillus jensenii TL2937 in particular. This review article emphasizes the cellular and molecular interactions of immunobiotics with IECs and immune cells through TLRs and their application for improving animal and human health.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis.
- Record: found
- Abstract: found
- Article: not found
Microbiota regulates immune defense against respiratory tract influenza A virus infection.
- Record: found
- Abstract: found
- Article: not found