- Record: found
- Abstract: found
- Article: found
Flagella at the Host-Microbe Interface: Key Functions Intersect With Redundant Responses
Read this article at
Abstract
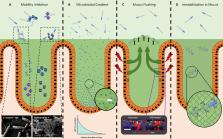
Many bacteria and other microbes achieve locomotion via flagella, which are organelles that function as a swimming motor. Depending on the environment, flagellar motility can serve a variety of beneficial functions and confer a fitness advantage. For example, within a mammalian host, flagellar motility can provide bacteria the ability to resist clearance by flow, facilitate access to host epithelial cells, and enable travel to nutrient niches. From the host’s perspective, the mobility that flagella impart to bacteria can be associated with harmful activities that can disrupt homeostasis, such as invasion of epithelial cells, translocation across epithelial barriers, and biofilm formation, which ultimately can decrease a host’s reproductive fitness from a perspective of natural selection. Thus, over an evolutionary timescale, the host developed a repertoire of innate and adaptive immune countermeasures that target and mitigate this microbial threat. These countermeasures are wide-ranging and include structural components of the mucosa that maintain spatial segregation of bacteria from the epithelium, mechanisms of molecular recognition and inducible responses to flagellin, and secreted effector molecules of the innate and adaptive immune systems that directly inhibit flagellar motility. While much of our understanding of the dynamics of host-microbe interaction regarding flagella is derived from studies of enteric bacterial pathogens where flagella are a recognized virulence factor, newer studies have delved into host interaction with flagellated members of the commensal microbiota during homeostasis. Even though many aspects of flagellar motility may seem innocuous, the host’s redundant efforts to stop bacteria in their tracks highlights the importance of this host-microbe interaction.
Related collections
Most cited references124
- Record: found
- Abstract: found
- Article: not found
The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system.
- Record: found
- Abstract: found
- Article: not found
Immunological aspects of intestinal mucus and mucins.
- Record: found
- Abstract: found
- Article: not found