- Record: found
- Abstract: found
- Article: found
Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species
Read this article at
Abstract
Abstract
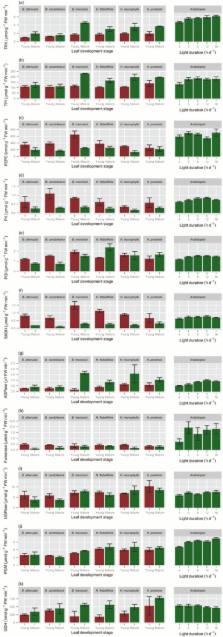
Proteaceae species in south-western Australia occur on phosphorus- (P) impoverished soils. Their leaves contain very low P levels, but have relatively high rates of photosynthesis. We measured ribosomal RNA (rRNA) abundance, soluble protein, activities of several enzymes and glucose 6-phosphate (Glc6P) levels in expanding and mature leaves of six Proteaceae species in their natural habitat. The results were compared with those for Arabidopsis thaliana. Compared with A. thaliana, immature leaves of Proteaceae species contained very low levels of rRNA, especially plastidic rRNA. Proteaceae species showed slow development of the photosynthetic apparatus (‘delayed greening’), with young leaves having very low levels of chlorophyll and Calvin–Benson cycle enzymes. In mature leaves, soluble protein and Calvin–Benson cycle enzyme activities were low, but Glc6P levels were similar to those in A. thaliana. We propose that low ribosome abundance contributes to the high P efficiency of these Proteaceae species in three ways: (1) less P is invested in ribosomes; (2) the rate of growth and, hence, demand for P is low; and (3) the especially low plastidic ribosome abundance in young leaves delays formation of the photosynthetic machinery, spreading investment of P in rRNA. Although Calvin–Benson cycle enzyme activities are low, Glc6P levels are maintained, allowing their effective use.
Related collections
Most cited references153
- Record: found
- Abstract: not found
- Book: not found
R: A language and environment for statistical computing
- Record: found
- Abstract: found
- Article: not found
From tropics to tundra: global convergence in plant functioning.
- Record: found
- Abstract: found
- Article: not found