- Record: found
- Abstract: found
- Article: found
Reproducing the dopamine pathophysiology of schizophrenia and approaches to ameliorate it: a translational imaging study with ketamine
Read this article at
Abstract
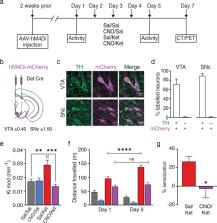
Patients with schizophrenia show increased striatal dopamine synthesis capacity in imaging studies. The mechanism underlying this is unclear but may be due to N-methyl-D-aspartate receptor (NMDAR) hypofunction and parvalbumin (PV) neuronal dysfunction leading to disinhibition of mesostriatal dopamine neurons. Here, we develop a translational mouse model of the dopamine pathophysiology seen in schizophrenia and test approaches to reverse the dopamine changes. Mice were treated with sub-chronic ketamine (30 mg/kg) or saline and then received in vivo positron emission tomography of striatal dopamine synthesis capacity, analogous to measures used in patients. Locomotor activity was measured using the open-field test. In vivo cell-type-specific chemogenetic approaches and pharmacological interventions were used to manipulate neuronal excitability. Immunohistochemistry and RNA sequencing were used to investigate molecular mechanisms. Sub-chronic ketamine increased striatal dopamine synthesis capacity (Cohen’s d = 2.5 ) and locomotor activity. These effects were countered by inhibition of midbrain dopamine neurons, and by activation of PV interneurons in pre-limbic cortex and ventral subiculum of the hippocampus. Sub-chronic ketamine reduced PV expression in these cortical and hippocampal regions. Pharmacological intervention with SEP-363856, a novel psychotropic agent with agonism at trace amine receptor 1 (TAAR1) and 5-HT 1A receptors but no appreciable action at dopamine D 2 receptors, significantly reduced the ketamine-induced increase in dopamine synthesis capacity. These results show that sub-chronic ketamine treatment in mice mimics the dopaminergic alterations in patients with psychosis, that this requires activation of midbrain dopamine neurons, and can be ameliorated by activating PV interneurons and by a TAAR1/5-HT 1A agonist. This identifies novel therapeutic approaches for targeting presynaptic dopamine dysfunction in patients with schizophrenia and effects of ketamine relevant to its therapeutic use for treating major depression.
Related collections
Most cited references117
- Record: found
- Abstract: found
- Article: not found
TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions
- Record: found
- Abstract: found
- Article: not found
Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010
- Record: found
- Abstract: found
- Article: not found