- Record: found
- Abstract: found
- Article: found
TGF-β1 modulates temozolomide resistance in glioblastoma via altered microRNA processing and elevated MGMT
Read this article at
Abstract
Background
Our previous studies have indicated that miR-198 reduces cellular methylguanine DNA methyltransferase (MGMT) levels to enhance temozolomide sensitivity. Transforming growth factor beta 1 (TGF-β1) switches off miR-198 expression by repressing K-homology splicing regulatory protein (KSRP) expression in epidermal keratinocytes. However, the underlying role of TGF-β1 in temozolomide resistance has remained unknown.
Methods
The distribution of KSRP was detected by western blotting and immunofluorescence. Microarray analysis was used to compare the levels of long noncoding RNAs (lncRNAs) between TGF-β1–treated and untreated cells. RNA immunoprecipitation was performed to verify the relationship between RNAs and KSRP. Flow cytometry and orthotopic and subcutaneous xenograft tumor models were used to determine the function of TGF-β1 in temozolomide resistance.
Results
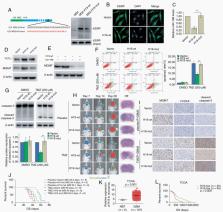
Overexpression of TGF-β1 contributed to temozolomide resistance in MGMT promoter hypomethylated glioblastoma cells in vitro and in vivo. TGF-β1 treatment reduced cellular MGMT levels through suppressing the expression of miR-198. However, TGF-β1 upregulation did not affect KSRP expression in glioma cells. We identified and characterized 2 lncRNAs (H19 and HOXD-AS2) that were upregulated by TGF-β1 through Smad signaling. H19 and HOXD-AS2 exhibited competitive binding to KSRP and prevented KSRP from binding to primary miR-198, thus decreasing miR-198 expression. HOXD-AS2 or H19 upregulation strongly promoted temozolomide resistance and MGMT expression. Moreover, KSRP depletion abrogated the effects of TGF-β1 and lncRNAs on miR-198 and MGMT. Finally, we found that patients with low levels of TGF-β1 or lncRNA expression benefited from temozolomide therapy.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma
- Record: found
- Abstract: found
- Article: not found