- Record: found
- Abstract: found
- Article: found
A low-carbohydrate high-fat diet increases weight gain and does not improve glucose tolerance, insulin secretion or β-cell mass in NZO mice
Read this article at
Abstract
Background/Objectives:
Dietary guidelines for the past 20 years have recommended that dietary fat should be minimized. In contrast, recent studies have suggested that there could be some potential benefits for reducing carbohydrate intake in favor of increased fat. It has also been suggested that low-carbohydrate diets be recommended for people with type 2 diabetes. However, whether such diets can improve glycemic control will likely depend on their ability to improve β-cell function, which has not been studied. The objective of the study was to assess whether a low-carbohydrate and therefore high-fat diet (LCHFD) is beneficial for improving the endogenous insulin secretory response to glucose in prediabetic New Zealand Obese (NZO) mice.
Methods:
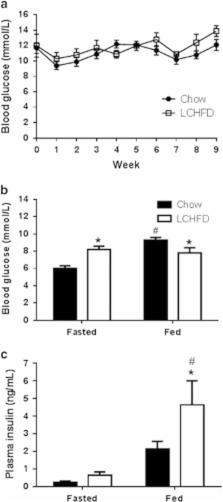
NZO mice were maintained on either standard rodent chow or an LCHFD from 6 to 15 weeks of age. Body weight, food intake and blood glucose were assessed weekly. Blood glucose and insulin levels were also assessed after fasting and re-feeding and during an oral glucose tolerance test. The capacity of pancreatic β-cells to secrete insulin was assessed in vivo with an intravenous glucose tolerance test. β-Cell mass was assessed in histological sections of pancreata collected at the end of the study.
Results:
In NZO mice, an LCHFD reduced plasma triglycerides ( P=0.001) but increased weight gain ( P<0.0001), adipose tissue mass ( P=0.0015), high-density lipoprotein cholesterol ( P=0.044) and exacerbated glucose intolerance ( P=0.013). Although fasting insulin levels tended to be higher ( P=0.08), insulin secretory function in LCHFD-fed mice was not improved ( P=0.93) nor was β-cell mass ( P=0.75).
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes.
- Record: found
- Abstract: found
- Article: not found
A Randomized Trial of a Low-Carbohydrate Diet for Obesity
- Record: found
- Abstract: found
- Article: not found