- Record: found
- Abstract: found
- Article: found
SecurAstaP trial: securement with SecurAcath versus StatLock for peripherally inserted central catheters, a randomised open trial
Read this article at
Abstract
Design, setting, participants
A parallel-group, open-label, randomised controlled trial in patients who are in need for a peripherally inserted central catheter insertion in one teaching hospital in Belgium. The follow-up lasted 180 days or until catheter removal, whatever came first. A computer generated table was used to allocate devices. Randomised patients were 105 adults (StatLock, n=53; SecurAcath, n=52) and primary analysis was based on all patients (n=92) with time measurements (StatLock, n=43; SecurAcath, n=49).
Interventions
StatLock which has to be changed weekly versus SecurAcath which could remain in place for the complete catheter dwell time.
Main outcome measure
Needed time for the dressing change at each dressing change (SecurAcath) or at each dressing change combined with the change of the securement device (StatLock).
Results
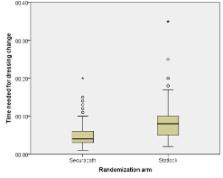
Median time needed for dressing change was 7.3 min (95% CI 6.4 min to 8.3 min) in the StatLock group and in the SecurAcath group 4.3 min (95% CI 3.8 min to 4.9 min) (P<0.0001). The time in the SecurAcath group was reduced with 41% (95% CI 29% to 51%). Incidence rates of migration, dislodgement and catheter-related bloodstream infection were comparable across groups. Pain scores were higher with SecurAcath than with StatLock at insertion (P=0.02) and at removal (P<0.001) and comparable during dressing change (P=0.38) and during dwell time (P=0.995). User-friendliness was scored at insertion and removal. All statements regarding the user-friendliness were scored significantly higher for StatLock than for SecurAcath (P<0.05). Only for the statement regarding the recommending routine use of the device, which was asked at removal, no difference was found between the two devices (P=0.32).
Related collections
Most cited references8
- Record: found
- Abstract: found
- Article: not found
Dressing and securement for central venous access devices (CVADs): A Cochrane systematic review
- Record: found
- Abstract: found
- Article: not found
Sutureless securement device reduces complications of peripherally inserted central venous catheters.
- Record: found
- Abstract: found
- Article: not found