- Record: found
- Abstract: found
- Article: not found
Direct Determination of Resonance Energy Transfer in Photolyase: Structural Alignment for the Functional State
Read this article at
Abstract

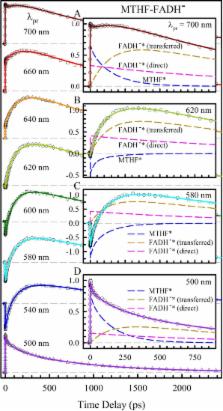
Photoantenna is essential to energy transduction in photoinduced biological machinery. A photoenzyme, photolyase, has a light-harvesting pigment of methenyltetrahydrofolate (MTHF) that transfers its excitation energy to the catalytic flavin cofactor FADH¯ to enhance DNA-repair efficiency. Here we report our systematic characterization and direct determination of the ultrafast dynamics of resonance energy transfer from excited MTHF to three flavin redox states in E. coli photolyase by capturing the intermediates formed through the energy transfer and thus excluding the electron-transfer quenching pathway. We observed 170 ps for excitation energy transferring to the fully reduced hydroquinone FADH¯, 20 ps to the fully oxidized FAD, and 18 ps to the neutral semiquinone FADH •, and the corresponding orientation factors (κ 2) were determined to be 2.84, 1.53 and 1.26, respectively, perfectly matching with our calculated theoretical values. Thus, under physiological conditions and over the course of evolution, photolyase has adopted the optimized orientation of its photopigment to efficiently convert solar energy for repair of damaged DNA.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Long-range resonance energy transfer in molecular systems.
- Record: found
- Abstract: found
- Article: not found
