- Record: found
- Abstract: found
- Article: found
Interactions with M Cells and Macrophages as Key Steps in the Pathogenesis of Enterohemorragic Escherichia coli Infections
Read this article at
Abstract
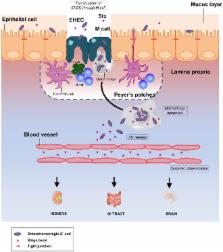
Enterohemorrhagic Escherichia coli (EHEC) are food-borne pathogens that can cause serious infections ranging from diarrhea to hemorrhagic colitis (HC) and hemolytic-uremic syndrome (HUS). Translocation of Shiga-toxins (Stx) from the gut lumen to underlying tissues is a decisive step in the development of the infection, but the mechanisms involved remain unclear. Many bacterial pathogens target the follicle-associated epithelium, which overlies Peyer's patches (PPs), cross the intestinal barrier through M cells and are captured by mucosal macrophages. Here, translocation across M cells, as well as survival and proliferation of EHEC strains within THP-1 macrophages were investigated using EHEC O157:H7 reference strains, isogenic mutants, and 15 EHEC strains isolated from HC/HUS patients. We showed for the first time that E. coli O157:H7 strains are able to interact in vivo with murine PPs, to translocate ex vivo through murine ileal mucosa with PPs and across an in vitro human M cell model. EHEC strains are also able to survive and to produce Stx in macrophages, which induce cell apoptosis and Stx release. In conclusion, our results suggest that the uptake of EHEC by M cells and underlying macrophages in the PP may be a critical step in Stx translocation and release in vivo. A new model for EHEC infection in humans is proposed that could help in a fuller understanding of EHEC-associated diseases.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections.
- Record: found
- Abstract: found
- Article: not found
Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response.
- Record: found
- Abstract: found
- Article: not found