- Record: found
- Abstract: found
- Article: found
The Prognostic Role of Baseline Metabolic Tumor Burden and Systemic Inflammation Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223: A Proof of Concept Study
Read this article at
Abstract
Simple Summary
Radium-223 is an alpha-emitting radioisotope that selectively binds to increased bone turnover areas, such as metastatic sites, acting as a bone-seeking calcium mimetic drug. Its therapeutic function in metastatic castration-resistant prostate cancer patients relies on its capability to prolong overall survival, improve quality of life, and delay the first skeletal-related event. However, in the last few years, many studies showed that the survival benefit in the real-life patients might be lower than that initially reported, probably due to a suboptimal selection of patients with poorer prognostic clinical characteristics. In this scenario, it has emerged the urgent need for the identification of reliable biomarkers able to potentially identify patients most likely to benefit from Radium-223 since baseline. With this aim, this preliminary study is the first to combine the prognostic power of baseline FDG-PET/CT and systemic inflammation indexes in a cohort of metastatic castration-resistant prostate cancer patients undergoing Radium-223 administration.
Abstract
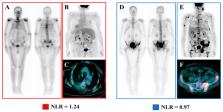
Over the last years has emerged the urgent need for the identification of reliable prognostic biomarkers able to potentially identify metastatic castration-resistant prostate cancer (mCRPC) patients most likely to benefit from Radium-223 (Ra-223) since baseline. In the present monocentric retrospective study, we analyzed the prognostic power of systemic inflammation biomarkers and 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG-PET)-derived parameters and their potential interplay in this clinical setting. The following baseline laboratory parameters were collected in 59 mCRPC patients treated with Ra-223: neutrophil-to-lymphocyte ratio (NLR), derived NLR (dNLR), lymphocyte-to-monocyte ratio (LMR), platelets-to-lymphocyte ratio (PLR), and systemic inflammation index (SII), while maximum Standardized Uptake Value, Metabolic Tumor Volume (MTV), and Total Lesion Glycolysis (TLG) were calculated in the 48 of them submitted to baseline FDG-PET. At the univariate analysis, NLR, dNLR, MTV, and TLG were able to predict the overall survival (OS). However, only NLR and MTV were independent predictors of OS at the multivariate analysis. Additionally, the occurrence of both increased NLR and MTV at baseline identified mCRPC patients at higher risk for lower long-term survival after treatment with Ra-223. In conclusion, the degree of systemic inflammation, the quantification of the metabolically active tumor burden and their combination might represent potentially valuable tools for identifying mCRPC patients who are most likely to benefit from Ra-223. However, further studies are needed to reproduce these findings in larger settings.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: found
Hallmarks of Cancer: The Next Generation
- Record: found
- Abstract: found
- Article: not found