- Record: found
- Abstract: found
- Article: found
Interactome determination of a Long Noncoding RNA implicated in Embryonic Stem Cell Self-Renewal
Read this article at
Abstract
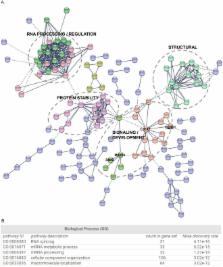
Long noncoding RNAs (lncRNAs) constitute a significant fraction of mammalian transcriptomes and they have emerged as intricate regulators of many biological processes. Their broad capacity to adopt diverse structures facilitates their involvement in the transcriptional, translational and signaling processes that are central to embryonic stem (ES) cell self-renewal and pluripotency. While lncRNAs have been implicated in ES cell maintenance, detailed analyses of those that show significant expression in ES cells is largely absent. Moreover, cooperative molecular relationships that facilitate lncRNA action are poorly understood. Cyrano is a developmentally important lncRNA, and in ES cells, it supports gene expression network maintenance, cell adhesion and cell survival. We have interrogated the interactome of Cyrano to identify protein partners and find that Cyrano is involved in multiple protein networks. We identify a developmentally important cell-signaling hub and find STAT3 as a candidate through which Cyrano can function to reinforce self-renewal of ES cells. Based on commonalities between ES cells and cancer cells, we postulate such functional interactions may support cell proliferation, cell identity and adhesion characteristics in rapidly proliferating cell types. The interactome data will therefore provide a resource for further investigations into interactions that regulate Cyrano or mediate its function.
Related collections
Most cited references11

- Record: found
- Abstract: found
- Article: found
BigWig and BigBed: enabling browsing of large distributed datasets
- Record: found
- Abstract: found
- Article: not found
Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells.
- Record: found
- Abstract: found
- Article: not found