- Record: found
- Abstract: found
- Article: found
Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells
Read this article at
Abstract
Background:
Glioblastoma multiforme (GBM) cells are resistant to anticancer drugs. Cancer stem cells (CSCs) are a key mediator of chemoresistance. We have reported that disulfiram (DS), an aldehyde dehydrogenase (ALDH) inhibitor, targets breast CSC-like cells. In this study, the effect of DS and combination of DS and gemcitabine (dFdC) on GBM cells and GBM stem-like cells was investigated.
Methods:
1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT), combination index (CI)-isobologram, western blot, luciferase reporter gene assay, electrophoretic mobility-shift assay and ALDH analysis were used in this study.
Results:
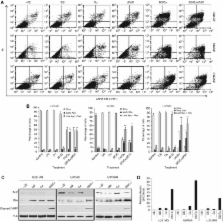
Disulfiram is cytotoxic in GBM cell lines in a copper (Cu)-dependent manner. Disulfiram/copper enhances the cytotoxicity of dFdC. Combination index-isobologram analysis indicates a synergistic effect between DS/Cu and dFdC. Disulfiram/copper induces reactive oxygen species (ROS), activates JNK and p38 pathways and inhibits nuclear factor-kappa B activity in GBM cell lines. Disulfiram/copper may trigger intrinsic apoptotic pathway via modulation of the Bcl2 family. Disulfiram/copper abolishes stem-like cell population in GBM cell lines.
Conclusion:
Our findings indicate that the cytotoxicity of DS/Cu and the enhancing effect of DS/Cu on the cytotoxicity of dFdC in GBM stem-like cells may be caused by induction of ROS and inhibition of both ALDH and the NFkB pathway. Both DS and dFdC can traverse the blood–brain barrier. Further study may lead them into GBM chemotherapy.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors.
- Record: found
- Abstract: found
- Article: not found
NF-kappaB activation by reactive oxygen species: fifteen years later.
- Record: found
- Abstract: found
- Article: not found