- Record: found
- Abstract: found
- Article: found
SHARPIN Is Essential for Cytokine Production, NF-κB Signaling, and Induction of Th1 Differentiation by Dendritic Cells
Read this article at
Abstract
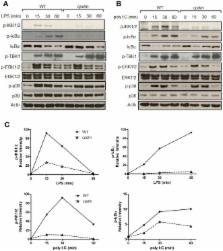
Spontaneous mutations of the Sharpin ( SHANK-associated RH domain-interacting protein, other aliases: Rbckl1, Sipl1) gene in mice result in systemic inflammation that is characterized by chronic proliferative dermatitis and dysregulated secretion of T helper1 (Th1) and Th2 cytokines. The cellular and molecular mechanisms underlying this inflammatory phenotype remain elusive. Dendritic cells may contribute to the initiation and progression of the phenotype of SHARPIN-deficient mice because of their pivotal role in innate and adaptive immunity. Here we show by flow cytometry that SHARPIN- deficiency did not alter the distribution of different DC subtypes in the spleen. In response to TOLL-like receptor (TLR) agonists LPS and poly I:C, cultured bone marrow-derived dendritic cells (BMDC) from WT and mutant mice exhibited similar increases in expression of co-stimulatory molecules CD40, CD80, and CD86. However, stimulated SHARPIN-deficient BMDC had reduced transcription and secretion of pro-inflammatory mediators IL6, IL12P70, GMCSF, and nitric oxide. Mutant BMDC had defective activation of NF-κB signaling, whereas the MAPK1/3 (ERK1/2) and MAPK11/12/13/14 (p38 MAP kinase isoforms) and TBK1 signaling pathways were intact. A mixed lymphocyte reaction showed that mutant BMDC only induced a weak Th1 immune response but stimulated increased Th2 cytokine production from allogeneic naïve CD4 + T cells. In conclusion, loss of Sharpin in mice significantly affects the immune function of DC and this may partially account for the systemic inflammation and Th2-biased immune response.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
MAP kinases in the immune response.
- Record: found
- Abstract: found
- Article: not found
SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis.
- Record: found
- Abstract: found
- Article: not found