- Record: found
- Abstract: found
- Article: found
The odontoblastic differentiation of dental mesenchymal stem cells: molecular regulation mechanism and related genetic syndromes
Read this article at
Abstract
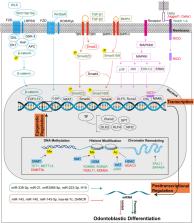
Dental mesenchymal stem cells (DMSCs) are multipotent progenitor cells that can differentiate into multiple lineages including odontoblasts, osteoblasts, chondrocytes, neural cells, myocytes, cardiomyocytes, adipocytes, endothelial cells, melanocytes, and hepatocytes. Odontoblastic differentiation of DMSCs is pivotal in dentinogenesis, a delicate and dynamic process regulated at the molecular level by signaling pathways, transcription factors, and posttranscriptional and epigenetic regulation. Mutations or dysregulation of related genes may contribute to genetic diseases with dentin defects caused by impaired odontoblastic differentiation, including tricho-dento-osseous (TDO) syndrome, X-linked hypophosphatemic rickets (XLH), Raine syndrome (RS), hypophosphatasia (HPP), Schimke immuno-osseous dysplasia (SIOD), and Elsahy-Waters syndrome (EWS). Herein, recent progress in the molecular regulation of the odontoblastic differentiation of DMSCs is summarized. In addition, genetic syndromes associated with disorders of odontoblastic differentiation of DMSCs are discussed. An improved understanding of the molecular regulation and related genetic syndromes may help clinicians better understand the etiology and pathogenesis of dentin lesions in systematic diseases and identify novel treatment targets.
Related collections
Most cited references260
- Record: found
- Abstract: found
- Article: not found
Functional Classification and Experimental Dissection of Long Noncoding RNAs
- Record: found
- Abstract: found
- Article: not found
Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities.
- Record: found
- Abstract: found
- Article: not found