- Record: found
- Abstract: found
- Article: found
FGF19 promotes cell autophagy and cisplatin chemoresistance by activating MAPK signaling in ovarian cancer
Read this article at
Abstract
Background
Chemotherapy is one of the primary treatments for ovarian cancer patients. Autophagy has been linked to chemotherapy resistance in tumor cells. Recent studies have suggested that fibroblast growth factor 19 (FGF19) may be involved in the onset and progression of malignancies. However, the relationship between FGF19 and autophagy in ovarian cancer is still unknown.
Methods
Next-generation sequencing (NGS) was conducted to analyze gene mutation profiles of 62 cases of high grade serous ovarian cancer (HGSOC). Fluorescence in situ hybridization (FISH) was performed to validate the amplification of FGF19 in HGSOC tissues. Quantitative PCR (qPCR) and immunohistochemistry (IHC) were used to analyze the difference of FGF19 in mRNA and protein expression. Meanwhile, bioinformatics techniques were used to analyze the expression profiles of FGF19 and the correlation with prognosis. Besides, immunofluorescence, transmission electron microscopy and Cell Counting Kit 8 (CCK-8) were used to investigate the potential mechanisms.
Results
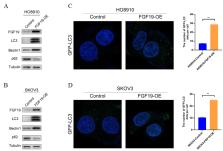
In this study, we found that FGF19 promotes cisplatin resistance in ovarian cancer cells by inducing autophagy. NGS analysis of 62 HGSOC cases identified a significantly amplified gene, FGF19. In addition, the expression level of FGF19 in ovarian cancer samples was higher than that in normal samples. FISH results showed a positive correlation between amplification and expression of FGF19. Knockdown of FGF19 inhibited the cell autophagy through decrease in the expression of LC3 and Beclin 1, and increase in the expression of SQSTM1/p62. Furthermore, we observed that p38 MAPK phosphorylation was down-regulated after FGF19 knockdown. IFN-γ, a potential p38 MAPK activator, counteracted the inhibition of cell autophagy and the anti-proliferation effect of cisplatin induced by FGF19 knockdown in ovarian cancer cells.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Autophagy in the pathogenesis of disease.
- Record: found
- Abstract: found
- Article: not found
Evolution of the Fgf and Fgfr gene families.
- Record: found
- Abstract: found
- Article: not found