- Record: found
- Abstract: found
- Article: not found
Bovine lactoferrin improves bone mass and microstructure in ovariectomized rats via OPG/RANKL/RANK pathway
Read this article at
Abstract
Aim:
Lactoferrin (LF), an 80-kDa iron-binding glycoprotein, is a pleiotropic factor found in colostrum, milk, saliva and epithelial cells of the exocrine glands. The aim of this study was to evaluate the effects of LF on the bones in ovariectomized (Ovx) rats and to identify the pathways that mediate the anabolic action of LF on the bones.
Methods:
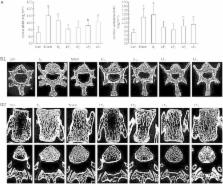
Female Sprague-Dawley rats (6-month-old) underwent ovariectomy, and were treated with different doses of LF (10, 100, 1000, and 2000 mg·kg −1·d −1, po) or with 7β-estradiol (0.1 mg·kg −1, im, each week) as the positive control. By the end of 6 month-treatments, the bone mass and microstructure in the rats were scanned by micro-computed tomography (micro-CT), and the bone metabolism was evaluated with specific markers, and the mRNA levels of osteoprotegerin (OPG) and the receptor-activator of nuclear factor κB ligand (RANKL) in femur were measured using qRT-PCR.
Results:
LF treatment dose-dependently elevated the bone volume (BV/TV), trabecular thickness (TbTh) and trabecular number (TbN), and reduced the trabecular separation (TbSp) in Ovx rats. Furthermore, higher doses of LF (1000 and 2000 mg·kg −1·d −1) significantly increased the bone mineral density (BMD) compared with the untreated Ovx rats. The higher doses of LF also significantly increased the serum levels of OC and BALP, and decreased the serum levels of β-CTx and NTX. LF treatment significantly increased the OPG mRNA levels, and suppressed the RANKL mRNA levels, and the RANKL/OPG mRNA ratio in Ovx rats.
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction.
- Record: found
- Abstract: found
- Article: not found
Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis.
- Record: found
- Abstract: found
- Article: not found
