- Record: found
- Abstract: found
- Article: found
Occludin, caveolin‐1, and Alix form a multi‐protein complex and regulate HIV‐1 infection of brain pericytes
Read this article at
Abstract
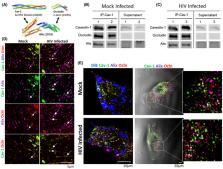
HIV‐1 enters the brain by altering properties of the blood‐brain barrier (BBB). Recent evidence indicates that among cells of the BBB, pericytes are prone to HIV‐1 infection. Occludin (ocln) and caveolin‐1 (cav‐1) are critical determinants of BBB integrity that can regulate barrier properties of the BBB in response to HIV‐1 infection. Additionally, Alix is an early acting endosomal factor involved in HIV‐1 budding from the cells. The aim of the present study was to evaluate the role of cav‐1, ocln, and Alix in HIV‐1 infection of brain pericytes. Our results indicated that cav‐1, ocln, and Alix form a multi‐protein complex in which they cross‐regulate each other's expression. Importantly, the stability of this complex was affected by HIV‐1 infection. Modifications of the complex resulted in diminished HIV‐1 infection and alterations of the cytokine profile produced by brain pericytes. These results identify a novel mechanism involved in HIV‐1 infection contributing to a better understanding of the HIV‐1 pathology and the associated neuroinflammatory responses.
Related collections
Most cited references67

- Record: found
- Abstract: found
- Article: found
The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis
- Record: found
- Abstract: found
- Article: not found
Central nervous system pericytes in health and disease.
- Record: found
- Abstract: found
- Article: not found