- Record: found
- Abstract: found
- Article: found
Ligand substitutions between ruthenium–cymene compounds can control protein versus DNA targeting and anticancer activity

Read this article at
Abstract
Ruthenium compounds have become promising alternatives to platinum drugs by displaying specific activities against different cancers and favourable toxicity and clearance properties. Nonetheless, their molecular targeting and mechanism of action are poorly understood. Here we study two prototypical ruthenium-arene agents—the cytotoxic antiprimary tumour compound [(η 6- p-cymene)Ru(ethylene-diamine)Cl]PF 6 and the relatively non-cytotoxic antimetastasis compound [(η 6- p-cymene)Ru(1,3,5-triaza-7-phosphaadamantane)Cl 2]—and discover that the former targets the DNA of chromatin, while the latter preferentially forms adducts on the histone proteins. Using a novel ‘atom-to-cell’ approach, we establish the basis for the surprisingly site-selective adduct formation behaviour and distinct cellular impact of these two chemically similar anticancer agents, which suggests that the cytotoxic effects arise largely from DNA lesions, whereas the protein adducts may be linked to the other therapeutic activities. Our study shows promise for developing new ruthenium drugs, via ligand-based modulation of DNA versus protein binding and thus cytotoxic potential, to target distinguishing epigenetic features of cancer cells.
Abstract
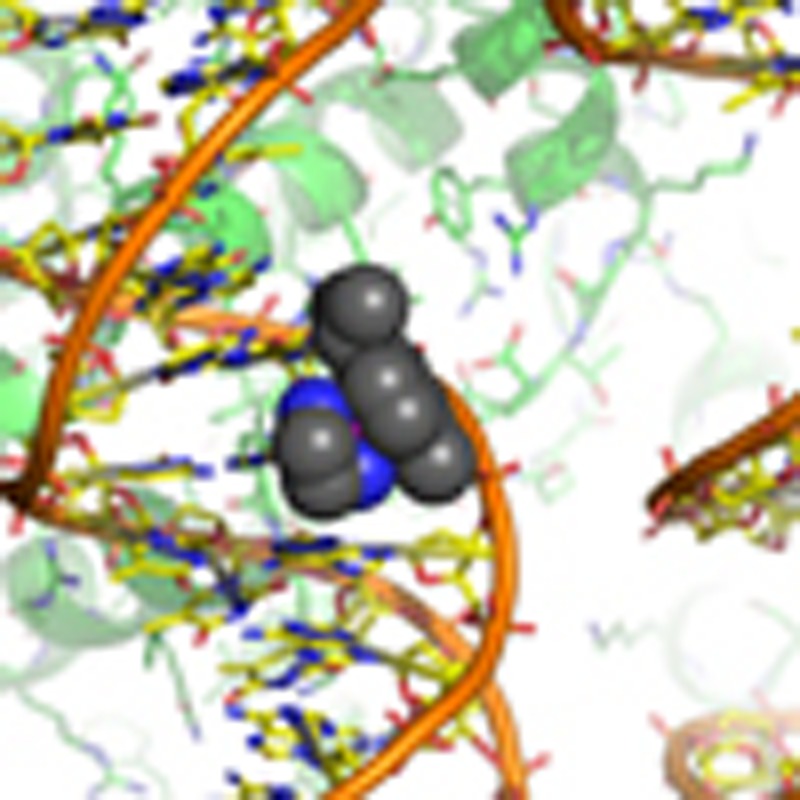 Ruthenium-cymene-based compounds are investigated as potential anticancer drugs. Here,
Adhireksan
et al. study two ruthenium-containing compounds with varying cytotoxicity and show that
differences in ligand structure may explain their activity and binding to different
subcellular targets.
Ruthenium-cymene-based compounds are investigated as potential anticancer drugs. Here,
Adhireksan
et al. study two ruthenium-containing compounds with varying cytotoxicity and show that
differences in ligand structure may explain their activity and binding to different
subcellular targets.
Related collections
Most cited references30
- Record: found
- Abstract: not found
- Article: not found
Generalized Gradient Approximation Made Simple.
- Record: found
- Abstract: found
- Article: not found
The NCI60 human tumour cell line anticancer drug screen.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.