- Record: found
- Abstract: found
- Article: found
Protracted Outbreak of Salmonella Newport Infections Linked to Ground Beef: Possible Role of Dairy Cows — 21 States, 2016–2017
research-article
Katherine E. Heiman Marshall , MPH
1
,
,
Mackenzie Tewell , MPH
2 ,
Selam Tecle , MPH
3 ,
Molly Leeper , MPH
1 ,
Jennifer Sinatra , DVM
4 ,
Bonnie Kissler , MPH
4 ,
Adrienne Fung , MPH
5 ,
Kerri Brown , MSPH
6 ,
Darlene Wagner , PhD
1 ,
Eija Trees , PhD
1 ,
Kelley B. Hise , MPH
1 ,
Vishnu Chaturvedi , PhD
7 ,
Linda K. Schlater , DVM
8 ,
Brenda R. Morningstar-Shaw , MS
8 ,
Laura Whitlock , MPH
1 ,
Kristin Holt , DVM
4 ,
Karen Becker , DVM
4 ,
Megin Nichols , DVM
1 ,
Ian T. Williams , PhD
1 ,
Michael Jhung , MD
1 ,
Matthew E. Wise , PhD
1 ,
Laura Gieraltowski , PhD
1
20 April 2018
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
In January 2017, CDC identified a cluster of Salmonella enterica serotype Newport
infections with isolates sharing an indistinguishable pulsed-field gel electrophoresis
(PFGE) pattern, JJPX01.0010 (pattern 10), through PulseNet, the national molecular
subtyping network for foodborne disease surveillance. This report summarizes the investigation
by CDC, state and local health and agriculture departments, and the U.S. Department
of Agriculture’s Food Safety and Inspection Service (USDA-FSIS) and discusses the
possible role of dairy cows as a reservoir for strains of Salmonella that persistently
cause human illness. This investigation combined epidemiologic and whole genome sequencing
(WGS) data to link the outbreak to contaminated ground beef; dairy cows were hypothesized
to be the ultimate source of Salmonella contamination.
Epidemiologic Investigation
A case was defined as infection with Salmonella Newport with PFGE pattern 10 closely
related to the outbreak strain by WGS, with bacterial isolation during October 1,
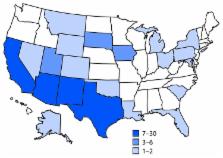
2016, through July 31, 2017. A total of 106 cases were identified in 21 states (Figure
1). Most illnesses ([72%]) were reported from southwestern states, including Arizona
(30), California (25), New Mexico (14), and Texas (seven). Illness onset dates ranged
from October 4, 2016, through July 19, 2017 (Figure 2). Patients ranged in age from
<1–88 years (median = 44 years), and 53 (50%) were female. Among 88 (83%) patients
with known outcomes, 42 (48%) were hospitalized, and one died.
FIGURE 1
Infections with the outbreak strain of Salmonella Newport (n = 106), by state of residence
— 21 states, October 2016–July 2017
The figure above is a map showing infections with the outbreak strain of Salmonella
Newport (n = 106), by state of residence in 21 states during October 2016–July 2017.
FIGURE 2
Isolates of the outbreak strain of Salmonella Newport from patients (n = 106), dairy
cattle* (n = 3), and leftover ground beef (n = 1) — 21 states, October 2016–July 2017
*The isolate collected from a dairy cow fetus in July 2016 is not displayed because
cases were reported during July–October 2016 but were not investigated as part of
this outbreak.
The figure above is a histogram showing isolates of the outbreak strain of Salmonella
Newport from patients (n = 106), dairy cattle* (n = 3), and leftover ground beef (n
= 1) in 21 states during October 2016–July 2017.
Initial interviews identified consumption of ground beef as a common exposure among
patients. A focused questionnaire was developed to collect detailed information on
ground beef exposure and to obtain shopper card information and receipts. Among 65
interviewed patients, 52 (80%) reported eating ground beef at home in the week before
illness began. This percentage was significantly higher than the 2006–2007 FoodNet
Population Survey, in which 40% of healthy persons reported eating ground beef at
home in the week before they were interviewed (p<0.001) (
1
). Among the 52 patients who ate ground beef at home, 31 (60%) reported that they
bought it or maybe bought it from multiple locations of two national grocery chains,
and 21 (40%) reported that they bought ground beef from locations of 15 other grocery
chains. Specific ground beef information was available for 35 patients. Among these,
15 (43%) purchased ground beef as chubs (rolls) of varying sizes (range = 2–10 lbs),
18 purchased it on a tray wrapped in plastic, and two purchased preformed hamburger
patties. Twenty-nine patients reported that they bought fresh ground beef, four bought
frozen ground beef, and four did not recall whether it was fresh or frozen when purchased.
When asked about ground beef preparation, 12 (36%) of 33 patients reported that they
definitely or possibly undercooked it.
Traceback Investigation
USDA-FSIS conducted traceback on ground beef purchased within 3 months of illness
onset for 11 patients who provided shopper card records or receipts. Approximately
20 ground beef suppliers belonging to at least 10 corporations were identified; 10
of the 11 records traced back to five company A slaughter/processing establishments,
seven of 11 traced back to five company B slaughter/processing establishments, and
four of 11 traced back to two company C slaughter/processing establishments.
Product and Animal Testing
Opened, leftover samples of ground beef from three patients’ homes were collected
for testing. All were purchased from one of two national grocery chains that had been
identified by a majority of patients. One sample, collected from ground beef removed
from its original packaging, yielded the outbreak strain. The other two samples did
not yield Salmonella.
The outbreak strain was also isolated from four New Mexico dairy cattle (Table). One
was collected from a spontaneously aborted fetus in July 2016, and one was isolated
from feces from a young calf in November 2016. The third isolate was identified by
searching the USDA Animal and Plant Health Inspection Service National Veterinary
Services Laboratory (USDA-APHIS NVSL) database for Salmonella Newport isolates collected
from cattle in Arizona, California, Texas, New Mexico, and Wisconsin during January
2016–March 2017. Eighteen Salmonella Newport isolates were identified, including 13
from Texas, three from New Mexico, and two from Wisconsin. The only Salmonella Newport
pattern 10 isolate identified was from a fecal sample from a New Mexico dairy cow
collected during November 2016. The fourth isolate was from a USDA-FSIS routine cattle
fecal sample collected at a Texas slaughter establishment in December 2016; USDA-FSIS
determined the sample was from a dairy cow and identified the New Mexico farm of origin.
Because of confidentiality practices, officials were not able to identify the farm
or farms of origin for the dairy cows associated with the other three samples or whether
the four dairy cows were associated with a single farm. None of the 11 patients with
information for traceback ate ground beef produced at the Texas slaughter establishment.
TABLE
Salmonella Newport pattern 10 isolates with the outbreak strain collected from dairy
cattle sourced from New Mexico, 2016
Isolate no.
Collection site
Isolation date
Sample source or reason for collection
1
Fetal tissue
Jul 7, 2016
Necropsy of cow fetus
2
Feces
Nov 14, 2016
Young calf
3
Feces
Nov 23, 2016
Cattle of unknown age collected because of infection*
4
Cecum
Dec 19, 2016
Routine sampling at slaughter facility in Texas; cow traced to New Mexico
Abbreviation: NVSL = USDA Animal and Plant Health Inspection Service National Veterinary
Services Laboratory.
*Because of the anonymity of samples from cows routinely tested by NVSL, it is possible
that this isolate is from the same sample as isolate 2.
Laboratory Investigation
Whole genome high-quality single nucleotide polymorphism (SNP) analysis* showed that
106 clinical isolates were closely related to each other genetically, to the four
dairy cattle isolates, and to the leftover ground beef isolate (range = 0–12 SNP differences),
suggesting that the Salmonella bacteria found in patients, ground beef, and dairy
cattle all shared a common source. Thirty-nine additional clinical isolates with PFGE
pattern 10 were determined to not be closely related and were excluded from the outbreak.
No antibiotic resistance was detected among three clinical isolates tested by CDC’s
National Antimicrobial Resistance Monitoring Laboratory.
†
Public Health Response
Because the USDA-FSIS traceback investigation did not converge on a common production
lot of ground beef or a single slaughter/processing establishment, and no ground beef
in the original packaging yielded the outbreak strain, a recall of specific product
was not requested. A public warning was not issued to consumers because specific,
actionable information was not available (e.g., a specific brand or type of ground
beef). Officials in New Mexico visited the dairy farm that was the source of the cow
at the Texas establishment and noted no concerns about conditions or practices. However,
this visit occurred late in the investigation, and conditions at the time of the visit
might not have represented those present immediately before and during the outbreak.
No samples from the environment or cows were collected during this visit.
Discussion
Epidemiologic and laboratory evidence indicated that contaminated ground beef was
the likely source of this protracted outbreak of Salmonella Newport infections. A
significantly higher percentage of patients than expected ate ground beef at home,
and a patient’s leftover ground beef yielded the outbreak strain. Dairy cows colonized
or infected with the outbreak strain before slaughter are hypothesized to be the ultimate
outbreak source. Most U.S. ground beef is produced from beef cattle; however, 18%
is produced from dairy cows (
2
). Dairy cows are sold for beef production through sale barns or directly to slaughter
establishments as they age or if their milk production is insufficient (
2
). Previous studies have demonstrated long-term persistence of Salmonella Newport
in dairy herds (
3
,
4
), and a 1987 Salmonella Newport outbreak was linked to contaminated ground beef from
slaughtered dairy cows (
5
). In the current outbreak, as has been observed in previous outbreaks, ground beef
purchases traced back to numerous lots and slaughter/processing establishments (
6
). One possible explanation is that dairy cows carrying a high Salmonella load that
overwhelmed antimicrobial interventions could have gone to multiple slaughter/processing
establishments (
7
), resulting in contamination of multiple brands and lots of ground beef. This might
explain the reason for failure to identify a single, specific source of contaminated
ground beef.
This investigation identified the outbreak strain only in samples from dairy cattle
from New Mexico. All four isolates from dairy cattle samples were closely related
genetically by WGS to isolates from patients, providing further evidence of a connection
between dairy cattle in New Mexico and the outbreak. The disproportionate geographic
distribution of cases in the U.S. Southwest, including New Mexico, also suggests a
possible regional outbreak source. Although limited in scope, the query of the USDA-APHIS
NVSL data identified the outbreak strain only from one New Mexico dairy cow (isolate
3), and the sample collection date was consistent with the timing of illnesses in
this outbreak. The overall prevalence and geographic distribution of the outbreak
strain in cattle is not known, and it is possible that cattle in states other than
New Mexico might have been infected or colonized with the outbreak strain.
This was a complex and challenging investigation for several reasons. First, the PFGE
pattern in the outbreak was not uncommon in PulseNet, making it difficult to distinguish
outbreak cases from sporadic illnesses associated with the same Salmonella Newport
pattern. WGS analysis provided more discriminatory power to refine the outbreak case
definition and excluded 39 cases of illness from the outbreak. However, sequencing
is not currently performed in real time for Salmonella, thereby slowing the process
of determining which cases were likely outbreak-associated. In addition, a direct
pathway linking outbreak cases to dairy cows infected with the outbreak strain of
Salmonella Newport could not be established. This is because product traceback did
not converge on a single contaminated lot of ground beef, and investigators were unable
to ascertain a link between the beef slaughter/processing establishments identified
during traceback and the farms with dairy cows that yielded the outbreak strain. Tracing
back ground beef purchased by patients to slaughter/processing establishments requires
documentation such as receipts or shopper card records, and only 10% of patients had
this information available. For this outbreak, tracing back cows at slaughter/processing
establishments to the farm from which they originated was problematic because cows
were not systematically tracked from farm to slaughter/processing establishments.
Four points along the “farm to fork” continuum provide opportunities to prevent consumers
from becoming ill from contaminated ground beef. First, farms can implement good management
practices for cattle health, including vaccination, biosecurity (e.g., controlling
movement of persons and animals on farms, keeping a closed herd [so that no animals
on the farm are purchased, loaned to other farms, or have contact with other animals],
planning introduction of new animals and quarantining them, and performing microbiologic
testing of animals), and cleaning and disinfection measures to decrease Salmonella
burden in animals and the environments in which they reside, reducing the likelihood
that Salmonella will enter beef slaughter/processing establishments (
8
). Second, slaughter/processing establishments are required to maintain Hazard Analysis
and Critical Control Points systems to reduce Salmonella contamination as well as
slaughter and sanitary dressing procedures to prevent carcass contamination (
9
). Third, although Salmonella is not considered an adulterant in not-ready-to eat
(NRTE) meat products, USDA-FSIS likely will consider the product to be adulterated
when NRTE meat products are associated with an outbreak (
9
). Finally, consumers are advised to cook ground beef to 160°F (71°C) as measured
by a food thermometer to destroy any bacteria that might be present. Consumers are
also advised to wash hands, utensils, and surfaces often; separate and not cross-contaminate
foods; and refrigerate foods promptly and properly.
This investigation emphasizes the utility of WGS during outbreak investigations and
identifies the need for improvements in traceability from the consumer to the farm.
It also highlights the importance of continued evaluation of farm practices to help
reduce persistent Salmonella contamination on farms, contamination of ground beef,
and ultimately human illness.
Summary
What is already known about this topic?
Previous outbreaks of salmonellosis were linked to contaminated ground beef produced
from slaughtered dairy cows.
What is added by this report?
Contaminated ground beef was the likely source of a protracted outbreak of 106 Salmonella
Newport infections, 42 hospitalizations, and one death in 21 states during October
2016–July 2017. While no direct link was found, whole genome sequencing suggests dairy
cows were the ultimate outbreak source.
What are the implications for public health practice?
Foodborne outbreak investigations could be enhanced by improvements in the traceability
of cows from their originating farms or sale barns, through slaughter and processing
establishments, to ground beef sold to consumers.
Related collections
Most cited references4
- Record: found
- Abstract: found
- Article: not found
Chloramphenicol-resistant Salmonella newport traced through hamburger to dairy farms. A major persisting source of human salmonellosis in California.
J Spika, S. Waterman, G Hoo … (1987)

- Record: found
- Abstract: found
- Article: found
Outbreak of Salmonella Heidelberg Infections Linked to a Single Poultry Producer — 13 States, 2012–2013
- Record: found
- Abstract: found
- Article: not found
Long-term persistence of multi-drug-resistant Salmonella enterica serovar Newport in two dairy herds.
Robert H. Rice, Rowland Cobbold, R. Hancock … (2006)