- Record: found
- Abstract: found
- Article: found
Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs
Read this article at
Abstract
Objectives
Trypanosoma brucei drug transporters include the TbAT1/P2 aminopurine transporter and the high-affinity pentamidine transporter (HAPT1), but the genetic identity of HAPT1 is unknown. We recently reported that loss of T. brucei aquaglyceroporin 2 ( TbAQP2) caused melarsoprol/pentamidine cross-resistance (MPXR) in these parasites and the current study aims to delineate the mechanism by which this occurs.
Methods
The TbAQP2 loci of isogenic pairs of drug-susceptible and MPXR strains of T. brucei subspecies were sequenced. Drug susceptibility profiles of trypanosome strains were correlated with expression of mutated TbAQP2 alleles. Pentamidine transport was studied in T. brucei subspecies expressing TbAQP2 variants.
Results
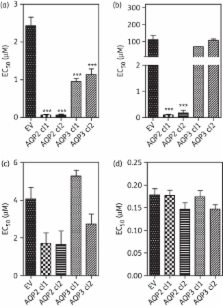
All MPXR strains examined contained TbAQP2 deletions or rearrangements, regardless of whether the strains were originally adapted in vitro or in vivo to arsenicals or to pentamidine. The MPXR strains and AQP2 knockout strains had lost HAPT1 activity. Reintroduction of TbAQP2 in MPXR trypanosomes restored susceptibility to the drugs and reinstated HAPT1 activity, but did not change the activity of TbAT1/P2. Expression of TbAQP2 sensitized Leishmania mexicana promastigotes 40-fold to pentamidine and >1000-fold to melaminophenyl arsenicals and induced a high-affinity pentamidine transport activity indistinguishable from HAPT1 by K m and inhibitor profile. Grafting the TbAQP2 selectivity filter amino acid residues onto a chimeric allele of AQP2 and AQP3 partly restored susceptibility to pentamidine and an arsenical.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
The genome of the African trypanosome Trypanosoma brucei.
- Record: found
- Abstract: found
- Article: not found
High-throughput decoding of anti-trypanosomal drug efficacy and resistance
- Record: found
- Abstract: not found
- Article: not found