- Record: found
- Abstract: found
- Article: found
Roles of hypoxia-inducible factor in hepatocellular carcinoma under local ablation therapies

Read this article at
Abstract
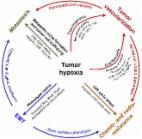
Hepatocellular carcinoma (HCC) is one of the most common digestive malignancies. HCC It ranges as the fifth most common cause of cancer mortality worldwide. While The prognosis of metastatic or advanced HCC is still quite poor. Recently, locoregional treatment, especially local ablation therapies, plays an important role in the treatment of HCC. Radiofrequency ablation (RFA) and high-intensity focused ultrasound (HIFU) ablation are the most common-used methods effective and feasible for treating HCC. However, the molecular mechanisms underlying the actions of ablation in the treatments for HCC and the HCC recurrence after ablation still are poorly understood. Hypoxia-inducible factor (HIF), the key gene switch for adaptive responses to hypoxia, has been found to play an essential role in the rapid aggressive recurrence of HCC after ablation treatment. In this review, we summarized the current evidence of the roles of HIF in the treatment of HCC with ablation. Fifteen relevant studies were included and further analyzed. Among them, three clinical studies suggested that HIF-1α might serve as a crucial role in the RAF treatment of HCC or the local recurrence of HCC after RFA. The remainder included experimental studies demonstrated that HIF-1, 2α might target the different molecules (e.g., BNIP3, CA-IX, and arginase-1) and signaling cascades (e.g., VEGFA/EphA2 pathway), constituting a complex network that promoted HCC invasion and metastasis after ablation. Currently, the inhibitors of HIF have been developed, providing important proof of targeting HIF for the prevention of HCC recurrence after IRFA and HIFU ablation. Further confirmation by prospective clinical and in-depth experimental studies is still warranted to illustrate the effects of HIF in HCC recurrence followed ablation treatment in the future.
Related collections
Most cited references136
- Record: found
- Abstract: found
- Article: found
The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy
- Record: found
- Abstract: found
- Article: not found
Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway.
- Record: found
- Abstract: found
- Article: not found