- Record: found
- Abstract: found
- Article: found
Orally administration of cerium oxide nanozyme for computed tomography imaging and anti-inflammatory/anti-fibrotic therapy of inflammatory bowel disease
Read this article at
Abstract
Background
Inflammatory bowel disease (IBD) is a chronic nonspecific disease with unknown etiology. Currently, the anti-inflammatory therapeutic approaches have achieved a certain extent of effects in terms of inflammation alleviation. Still, the final pathological outcome of intestinal fibrosis has not been effectively improved yet.
Results
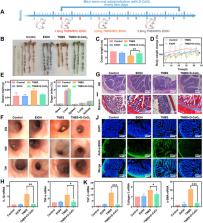
In this study, dextran-coated cerium oxide (D-CeO 2) nanozyme with superoxide dismutase (SOD) and catalase (CAT) activities was synthesized by chemical precipitation. Our results showed that D-CeO 2 could efficiently scavenge reactive oxide species (ROS) as well as downregulate the pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, and iNOS) to protect cells from H 2O 2-induced oxidative damage. Moreover, D-CeO 2 could suppress the expression of fibrosis-related gene levels, such as α-SMA, and Collagen 1/3, demonstrating the anti-fibrotic effect. In both TBNS- and DSS-induced colitis models, oral administration of D-CeO 2 in chitosan/alginate hydrogel alleviated intestinal inflammation, reduced colonic damage by scavenging ROS, and decreased inflammatory factor levels. Notably, our findings also suggested that D-CeO 2 reduced fibrosis-related cytokine levels, predicting a contribution to alleviating colonic fibrosis. Meanwhile, D-CeO 2 could also be employed as a CT contrast agent for noninvasive gastrointestinal tract (GIT) imaging.
Conclusion
We introduced cerium oxide nanozyme as a novel therapeutic approach with computed tomography (CT)-guided anti-inflammatory and anti-fibrotic therapy for the management of IBD. Collectively, without appreciable systemic toxicity, D-CeO 2 held the promise of integrated applications for diagnosis and therapy, pioneering the exploration of nanozymes with ROS scavenging capacity in the anti-fibrotic treatment of IBD.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Chemically induced mouse models of acute and chronic intestinal inflammation
- Record: found
- Abstract: found
- Article: not found