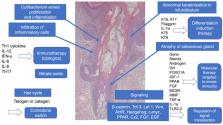
Keloids and hypertrophic scars are caused by cutaneous injury and irritation, including trauma, insect bite, burn, surgery, vaccination, skin piercing, acne, folliculitis, chicken pox, and herpes zoster infection. Notably, superficial injuries that do not reach the reticular dermis never cause keloidal and hypertrophic scarring. This suggests that these pathological scars are due to injury to this skin layer and the subsequent aberrant wound healing therein. The latter is characterized by continuous and histologically localized inflammation. As a result, the reticular layer of keloids and hypertrophic scars contains inflammatory cells, increased numbers of fibroblasts, newly formed blood vessels, and collagen deposits. Moreover, proinflammatory factors, such as interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor-α are upregulated in keloid tissues, which suggests that, in patients with keloids, proinflammatory genes in the skin are sensitive to trauma. This may promote chronic inflammation, which in turn may cause the invasive growth of keloids. In addition, the upregulation of proinflammatory factors in pathological scars suggests that, rather than being skin tumors, keloids and hypertrophic scars are inflammatory disorders of skin, specifically inflammatory disorders of the reticular dermis. Various external and internal post-wounding stimuli may promote reticular inflammation. The nature of these stimuli most likely shapes the characteristics, quantity, and course of keloids and hypertrophic scars. Specifically, it is likely that the intensity, frequency, and duration of these stimuli determine how quickly the scars appear, the direction and speed of growth, and the intensity of symptoms. These proinflammatory stimuli include a variety of local, systemic, and genetic factors. These observations together suggest that the clinical differences between keloids and hypertrophic scars merely reflect differences in the intensity, frequency, and duration of the inflammation of the reticular dermis. At present, physicians cannot (or at least find it very difficult to) control systemic and genetic risk factors of keloids and hypertrophic scars. However, they can use a number of treatment modalities that all, interestingly, act by reducing inflammation. They include corticosteroid injection/tape/ointment, radiotherapy, cryotherapy, compression therapy, stabilization therapy, 5-fluorouracil (5-FU) therapy, and surgical methods that reduce skin tension.