- Record: found
- Abstract: found
- Article: found
Characterization of Discrete Subpopulations of Progenitor Cells in Traumatic Human Extremity Wounds
Read this article at
Abstract
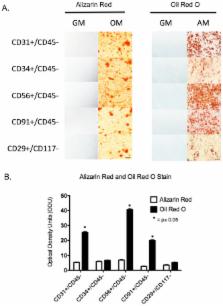
Here we show that distinct subpopulations of cells exist within traumatic human extremity wounds, each having the ability to differentiate into multiple cells types in vitro. A crude cell suspension derived from traumatized muscle was positively sorted for CD29, CD31, CD34, CD56 or CD91. The cell suspension was also simultaneously negatively sorted for either CD45 or CD117 to exclude hematopoietic stem cells. These subpopulations varied in terms their total numbers and their abilities to grow, migrate, differentiate and secrete cytokines. While all five subpopulations demonstrated equal abilities to undergo osteogenesis, they were distinct in their ability to undergo adipogenesis and vascular endotheliogenesis. The most abundant subpopulations were CD29+ and CD34+, which overlapped significantly. The CD29+ and CD34+ cells had the greatest proliferative and migratory capacity while the CD56+ subpopulation produced the highest amounts of TGFß1 and TGFß2. When cultured under endothelial differentiation conditions the CD29+ and CD34+ cells expressed VE-cadherin, Tie2 and CD31, all markers of endothelial cells. These data indicate that while there are multiple cell types within traumatized muscle that have osteogenic differentiation capacity and may contribute to bone formation in post-traumatic heterotopic ossification (HO), the major contributory cell types are CD29+ and CD34+, which demonstrate endothelial progenitor cell characteristics.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Conversion of vascular endothelial cells into multipotent stem-like cells.
- Record: found
- Abstract: found
- Article: not found
Expression of Cd34 and Myf5 Defines the Majority of Quiescent Adult Skeletal Muscle Satellite Cells
- Record: found
- Abstract: found
- Article: not found