- Record: found
- Abstract: found
- Article: found
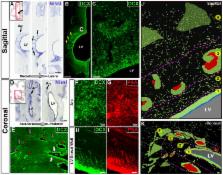
Extensive migration of young neurons into the infant human frontal lobe
Read this article at
Abstract
The first few months after birth, when a child begins to interact with the environment, are critical to human brain development. The human frontal lobe is important for social behavior and executive function; it has increased in size and complexity relative to other species, but the processes that have contributed to this expansion are unknown. Our studies of postmortem infant human brains revealed a collection of neurons that migrate and integrate widely into the frontal lobe during infancy. Chains of young neurons move tangentially close to the walls of the lateral ventricles and along blood vessels. These cells then individually disperse long distances to reach cortical tissue, where they differentiate and contribute to inhibitory circuits. Late-arriving interneurons could contribute to developmental plasticity, and the disruption of their postnatal migration or differentiation may underlie neurodevelopmental disorders.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Interneuron dysfunction in psychiatric disorders.
- Record: found
- Abstract: found
- Article: not found
Learning enhances adult neurogenesis in the hippocampal formation.
- Record: found
- Abstract: found
- Article: not found