- Record: found
- Abstract: found
- Article: not found
Reversal of IDO-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme
Abstract
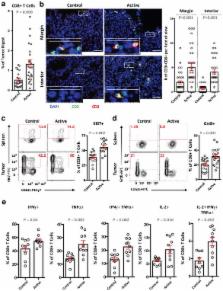
Elevated tryptophan (Trp) catabolism in the tumor microenvironment (TME) can mediate immune suppression by upregulation of IFNg-inducible indoleamine 2,3-dioxygenase (IDO1) and/or ectopic expression of the predominantly liver-restricted enzyme tryptophan 2,3-dioxygenase (TDO) 1– 5 . Whether these effects are due to Trp depletion in the TME or are mediated by the accumulation of the IDO1/TDO product Kynurenine (Kyn) remains controversial 5– 13 . Here we show that administration of a pharmacologically optimized enzyme (PEGylated Kynureninase) that degrades Kyn into immunologically inert, non-toxic and readily-cleared metabolites inhibits tumor growth. Enzyme treatment is associated with a marked increase in the tumor infiltration and proliferation of polyfunctional CD8 + lymphocytes. We show that PEG-Kynureninase administration has substantial therapeutic effects when combined with approved checkpoint inhibitors or with a cancer vaccine for the treatment of large B16-F10 melanoma, 4T1 breast carcinoma or CT26 colon carcinoma tumors. PEG-Kynureninase mediates prolonged depletion of Kyn in the TME and reverses the modulatory effects of IDO1/TDO upregulation in the tumor microenvironment.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels.
- Record: found
- Abstract: found
- Article: not found
Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion.
- Record: found
- Abstract: found
- Article: not found
