- Record: found
- Abstract: found
- Article: found
非小细胞肺癌患者 hOGG1基因启动子区域突变的研究 Translated title: Mutational Analysis of hOGG1 Gene Promoter in Patients with Non-small Cell Lung Cancer
Read this article at
Abstract
背景与目的
8-羟基鸟嘌呤DNA糖苷酶(8-hydroxygumine DNA glycosylase 1, OGG1)是一种DNA修复酶,可以从DNA切除修复8-羟基鸟嘌呤(8-dihydro-8-oxoguanine, 8-OH-G)。人类 OGG1基因( hOGG1)的多态性可能会改变酶的活性而影响个体修复损伤DNA的能力,促进癌变。然而, hOGG1基因启动子区域的突变与非小细胞肺癌(non-small cell lung cancer, NSCLC)的关系尚不明晰。我们拟探讨 hOGG1基因启动子区域的突变与NSCLC发生发展的潜在关系。
方法
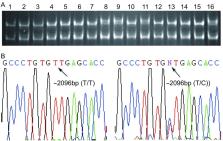
选取苏州大学附属第一医院2003年1月-2005年12月新鲜手术切除的40例NSCLC组织标本,采用PCR-SSCP和直接测序的方法检测NSCLC及其对应的癌旁组织中 hOGG1基因启动子区域的突变。
Translated abstract
8-hydroxygumine DNA glycosylase 1 (OGG1) is a DNA repair enzyme, which can repair damaged DNA by excising 8-dihydro-8-oxoguanine (8-OH-G). Polymorphisms in human OGG1 gene ( hOGG1) may alter glycosylase activity, thereby affects its repair to the damaged DNA, resulting in contribution to carcinogenesis. However, an association of genetic variants of hOGG1 promoter with non-small cell lung cancer (NSCLC) remains unclear. The present study aims to explore whether there are mutations in the promoter region of hOGG1 and the association of the potential genetic variants with NSCLC.
Forty lung cancer patients were enrolled from January, 2003 to December, 2005 in the first affiliated hospital of Soochow University. PCR-SSCP followed by direct sequencing were performed to detect mutations within the promoter region of the hOGG1 gene in NSCLC and corresponding paracancerous lung tissues.
No abnormal mutation was found in the promoter region of the hOGG1 gene in 40 patients with NSCLC. However, a SNP rs159153 in hOGG1 was significantly associated with higher TNM stage ( P=0.008). Moreover, lower frequency of lymph node metastasis was observed in smoker patients with NSCLC ( P=0.034).
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Polymorphisms in base-excision repair and nucleotide-excision repair genes in relation to lung cancer risk.
- Record: found
- Abstract: found
- Article: not found
The OGG1 gene encodes a repair enzyme for oxidatively damaged DNA and is involved in human carcinogenesis.
- Record: found
- Abstract: found
- Article: not found