- Record: found
- Abstract: found
- Article: found
PIWI-interacting RNA-36712 restrains breast cancer progression and chemoresistance by interaction with SEPW1 pseudogene SEPW1P RNA
Read this article at
Abstract
Background
Breast cancer is one of the most common malignancies and the major cause of cancer-related death in women. Although the importance of PIWI-interacting RNAs (piRNAs) in cancer has been increasingly recognized, few studies have been explored the functional mechanism of piRNAs in breast cancer development and progression.
Methods
We examined the top 20 highly expressed piRNAs based on the analysis of TCGA breast cancer data in two patient cohorts to test the roles of piRNAs in breast cancer. The effects of piRNA-36,712 on the malignant phenotypes and chemosensitivity of breast cancer cells were detected in vitro and in vivo. MS2-RIP and reporter gene assays were conducted to identify the interaction and regulation among piRNA-36,712, miRNAs and SEPW1P. Kaplan-Meier estimate with log-rank test was used to compare patient survival by different piRNA-36,712 expression levels.
Results
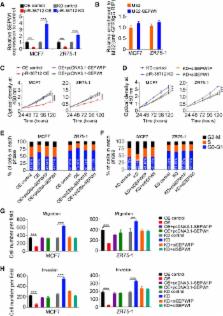
We found piRNA-36,712 level was significantly lower in breast cancer than in normal breast tissues and low level was correlated with poor clinical outcome in patients. Functional studies demonstrated that piRNA-36,712 interacts with RNAs produced by SEPW1P, a retroprocessed pseudogene of SEPW1, and subsequently inhibits SEPW1 expression through competition of SEPW1 mRNA with SEPW1P RNA for microRNA-7 and microRNA-324. We also found that higher SEPW1 expression due to downregulation of piRNA-36,712 in breast cancer may suppress P53, leading to the upregulated Slug but decreased P21 and E-cadherin levels, thus promoting cancer cell proliferation, invasion and migration. Furthermore, we found that piRNA-36,712 had synergistic anticancer effects with the paclitaxel and doxorubicin, two chemotherapeutic agents for breast cancer.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
A coding-independent function of gene and pseudogene mRNAs regulates tumour biology
- Record: found
- Abstract: found
- Article: not found
A germline-specific class of small RNAs binds mammalian Piwi proteins.
- Record: found
- Abstract: found
- Article: not found