- Record: found
- Abstract: found
- Article: found
Immunomodulatory Effect of Marine Cembrane-Type Diterpenoids on Dendritic Cells
Read this article at
Abstract
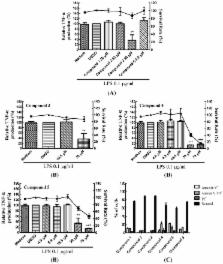
Dendritic cells (DCs) are antigen presenting cells, which can present antigens to T-cells and play an important role in linking innate and adaptive immunity. DC maturation can be induced by many stimuli, including pro-inflammatory cytokines and bacterial products, such as lipopolysaccharides (LPS). Here, we examined the immunomodulatory effects of marine cembrane compounds, (9 E,13 E)-5-acetoxy-6-hydroxy-9,13-dimethyl-3-methylene-3,3a,4,5,6,7,8,11,12,14a-decahydro-2 H-cyclotrideca[ b]furan-2-one ( 1), (9 E,13 E)-5-acetoxy-6-acetyl-9,13-dimethyl-3-methylene-3,3a,4,5,6,7,8,11,12,14a-decahydro-2 H-cyclotrideca[ b]furan-2-one ( 2), lobocrassin B ( 3), (−)14-deoxycrassin ( 4), cembranolide B ( 5) and 13-acetoxysarcocrassolide ( 6) isolated from a soft coral, Lobophytum crassum, on mouse bone marrow-derived dendritic cells (BMDCs). The results revealed that cembrane-type diterpenoids, especially lobocrassin B, effectively inhibited LPS-induced BMDC activation by inhibiting the production of TNF-α. Pre-treatment of BMDCs with Lobocrassin B for 1 h is essential to prohibit the following activation induced by various toll-like receptor (TLR) agonists, such as LPS, zymosan, lipoteichoic acid (LTA) and Pam2CSK4. Inhibition of NF-κB nuclear translocation by lobocrassin B, which is a key transcription factor for cytokine production in TLR signaling, was evident as assayed by high-content image analysis. Lobocrassin B attenuated DC maturation and endocytosis as the expression levels of MHC class II and the co-stimulatory molecules were downregulated, which may affect the function of DCs to initiate the T-cell responses. Thus, lobocrassin B may have the potential in treatment of immune dysregulated diseases in the future.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Taking dendritic cells into medicine.
- Record: found
- Abstract: found
- Article: not found