- Record: found
- Abstract: found
- Article: found
Konjac ceramide (kCer) regulates keratinocyte migration by Sema3A-like repulsion mechanism
Read this article at
Abstract
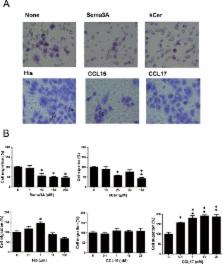
Previously, we proposed the following mechanism for konjac ceramide (kCer)-mediated neurite outgrowth inhibition: kCer binds to Nrp as a Sema3A agonist, resulting in Nrp1/PlexA complex formation and activation of the Sema3A signaling pathway to induce phosphorylation of CRMP2 and microtubule depolymerization. The Sema3A/Nrp1 signaling pathway is known to be also expressed in normal human keratinocytes. To determine whether kCer can function in human keratinocytes as it does in neurites, that is, if it can bind to Nrp1 in place of Sema3A, we studied the effect of kCer on HaCaT cell migration activity. Using a trans-well chamber assay, we compared the effects of Sema3A and kCer on serum-derived cell migration activity. kCer showed Sema3A-like suppression of cell migration activity and induction of cellular Cofilin phosphorylation. In addition, kCer and Sema3A inhibited histamine (His)-enhanced migration of immature HaCaT cells. We have demonstrated that kCer does not interact with histaime receptors H1R or H4R directly, but we speculate that kCer may transduce a signal downstream of the His signaling pathway.
Highlights
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Itch and nerve fibers with special reference to atopic dermatitis: therapeutic implications.
- Record: found
- Abstract: found
- Article: not found
Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A.
- Record: found
- Abstract: found
- Article: not found