- Record: found
- Abstract: found
- Article: found
N 6-methyladenosine (m 6A) methyltransferase METTL3-mediated LINC00680 accelerates osteoarthritis through m 6A/SIRT1 manner

Read this article at
Abstract
Increasing evidence suggest the biological roles of N 6-methyladenosine (m 6A) and long noncoding RNAs (lncRNAs) in the bone disease, especially osteoarthritis (OA). However, the interaction of m 6A and lncRNA in osteoarthritis is still unclear. Here, we found that a m 6A-related lncRNA LINC00680 upregulated in the OA tissue and IL-1β-induced isolated primary chondrocytes. Functionally, in IL-1β-induced chondrocytes, silencing of LINC00680 recovered the proliferation and repressed the extracellular matrix (ECM) degradation. Mechanistically, m 6A methyltransferase METTL3 combined tithe the m 6A site of LINC00680 to up-regulate its expression. Moreover, LINC00680 interacted with SIRT1 mRNA through binding at m 6A site on SIRT1 mRNA 3′-UTR, thereby enhancing the stability of SIRT1 mRNA. Overall, these findings exhibited a role of LINC00680/m 6A/SIRT1 mRNA complex in chondrocytes. Taken together, the present study intends to uncover the mechanism by which METTL3-mediated LINC00680 accelerates OA progression, which may provide novel insight for OA.
Abstract
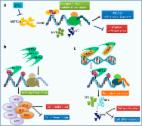
LINC00680/m6A/IGF2BP2/SIRT1 axis promotes the OA progression in chondrocyte.

Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: found
RNA N 6 -methyladenosine modification in cancers: current status and perspectives
- Record: found
- Abstract: found
- Article: not found