- Record: found
- Abstract: found
- Article: found
Developing H3K27M mutant selective radiosensitization strategies in diffuse intrinsic pontine glioma
Read this article at
Abstract
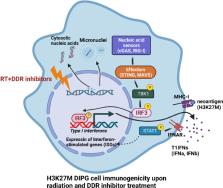
Diffuse intrinsic pontine glioma (DIPG) is a rare but highly lethal pediatric and adolescent tumor located in the pons of the brainstem. DIPGs harbor unique and specific pathological and molecular alterations, such as the hallmark lysine 27-to-methionine (H3K27M) mutation in histone H3, which lead to global changes in the epigenetic landscape and drive tumorigenesis. While fractionated radiotherapy, the current standard of care, improves symptoms and delays tumor progression, DIPGs inevitably recur, and despite extensive efforts chemotherapy-driven radiosensitization strategies have failed to improve survival. Advances in our understanding of the role of epigenetics in the cellular response to radiation-induced DNA damage, however, offer new opportunities to develop combinational therapeutic strategies selective for DIPGs expressing H3K27M. In this review, we provide an overview of preclinical studies that explore potential radiosensitization strategies targeting the unique epigenetic landscape of H3K27M mutant DIPG. We further discuss opportunities to selectively radiosensitize DIPG through strategic inhibition of the radiation-induced DNA damage response. Finally, we discuss the potential for using radiation to induce anti-tumor immune responses that may be potentiated in DIPG by radiosensitizing-therapeutic strategies.
Related collections
Most cited references108
- Record: found
- Abstract: found
- Article: found
The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary.
- Record: found
- Abstract: found
- Article: not found
The 2021 WHO Classification of Tumors of the Central Nervous System: a summary
- Record: found
- Abstract: found
- Article: not found