- Record: found
- Abstract: found
- Article: found
Opioid therapy duration before naldemedine treatment is a significant independent risk of diarrhea: a retrospective cohort study
Read this article at
Abstract
Background
The most common adverse event (AE) associated with opioid analgesics is opioid-induced constipation (OIC). Naldemedine (NAL) is widely used for the treatment of OIC. However, diarrhea has been reported as the most common treatment-emergent AE of NAL, and little is known about the risk factors associated with the development of diarrhea during NAL administration. This study examined the risk factors for NAL-induced diarrhea via a retrospective chart review of hospitalized patients.
Methods
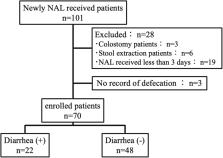
The data of 101 hospitalized adult patients who received NAL for the first time for the treatment of OIC at Mie University Hospital between June 2017 and December 2018 were extracted from electronic medical records. According to the inclusion and exclusion criteria, 70 of the 101 patients were enrolled in this study. Diarrhea was defined as “diarrhea” on the medical record within 2 weeks of NAL administration. Univariate and multivariate analyses were performed to identify risk factors for the development of diarrhea in patients receiving NAL.
Results
Twenty-two of the 70 patients enrolled (31%) developed diarrhea within 2 weeks of NAL administration. The median duration (range) of NAL treatment before diarrhea onset was 3 (1–12) days. Patients with diarrhea had a significantly longer duration of opioid therapy before NAL administration than patients without diarrhea ( P=0.002). Multivariate logistic regression analysis indicated that the independent risk factors for the development of NAL-induced diarrhea were NAL administration after more than 17 days of opioid therapy (odds ratio [OR]=7.539; P=0.016) and pancreatic cancer (OR=6.217; P=0.025). In fact, the incidence of diarrhea in patients who were administered NAL within a day of opioid therapy was significantly lower than that in patients who were administered NAL after more than 17 days of opioid therapy (13% vs. 54%, P=0.030).
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer.
- Record: found
- Abstract: found
- Article: not found
Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC.
- Record: found
- Abstract: not found
- Article: not found