- Record: found
- Abstract: found
- Article: found
Poldip2/Nox4 Mediates Lipopolysaccharide-Induced Oxidative Stress and Inflammation in Human Lung Epithelial Cells
Read this article at
Abstract
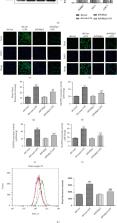
NADPH oxidase 4 (Nox4) is an important source of reactive oxygen species (ROS) production, and its expression is increased in lipopolysaccharide- (LPS-) stimulated lung epithelial cells. Polymerase δ-interacting protein 2 (Poldip2) has been proved to bind Nox4 and participates in oxidative stress and inflammation. However, the role of Poldip2/Nox4 in LPS-induced oxidative stress and inflammation in lung epithelial cells remains unclear. Cell viability was measured via MTT assays. The expression of Poldip2, Nox4, heme oxygenase-1 (HO-1), cyclooxygenase-2 (COX-2), AKT, and p-AKT was detected by Western blotting and/or immunofluorescence. Poldip2 and Nox4 interaction was analyzed via coimmunoprecipitation (Co-IP) assay. NADPH enzymatic activity and production of ROS, prostaglandin E2 (PGE2), tumor necrosis factor- α (TNF- α), and interleukin-1 β (IL-1 β) were assessed simultaneously. The small interfering RNA (siRNA) or plasmid targeting Nox4 was used to downregulate or upregulate Nox4, and the lentiviral vector encoding Poldip2 was used to downregulate or upregulate Poldip2. The present study demonstrated that LPS stimulation significantly increased the protein levels of Poldip2 and Nox4 and proved that Poldip2 interacted with Nox4 proved by Co-IP. Importantly, Poldip2 acted as an upstream regulator of Nox4. The increased expression of Nox4 and COX-2; NADPH enzymatic activity; production of ROS, PGE2, TNF- α, and IL-1 β; and decreased HO-1 expression were significantly suppressed by lentiviral Poldip2 shRNA downregulation but were increased by lentiviral upregulation of Poldip2. Furthermore, inhibiting of PI3K-AKT signaling notably attenuated LPS-induced Poldip2/Nox4 activation. Our study demonstrated that Poldip2 mediates LPS-induced oxidative stress and inflammation via interaction with Nox4 and was regulated by the PI3K-AKT signaling. Targeting Poldip2 could be a beneficial therapeutic strategy for the treatment of ALI.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis.
- Record: found
- Abstract: found
- Article: not found
Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases.
- Record: found
- Abstract: found
- Article: not found