- Record: found
- Abstract: found
- Article: found
Identification of the ESKAPE pathogens by mass spectrometric analysis of microbial membrane glycolipids
Read this article at
Abstract
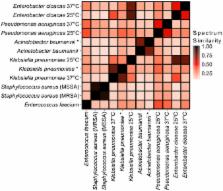
Rapid diagnostics that enable identification of infectious agents improve patient outcomes, antimicrobial stewardship, and length of hospital stay. Current methods for pathogen detection in the clinical laboratory include biological culture, nucleic acid amplification, ribosomal protein characterization, and genome sequencing. Pathogen identification from single colonies by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) analysis of high abundance proteins is gaining popularity in clinical laboratories. Here, we present a novel and complementary approach that utilizes essential microbial glycolipids as chemical fingerprints for identification of individual bacterial species. Gram-positive and negative bacterial glycolipids were extracted using a single optimized protocol. Extracts of the clinically significant ESKAPE pathogens: E nterococcus faecium, S taphylococcus aureus, K lebsiella pneumoniae, A cinetobacter baumannii, P seudomonas aeruginosa, and E nterobacter spp. were analyzed by MALDI-TOF-MS in negative ion mode to obtain glycolipid mass spectra. A library of glycolipid mass spectra from 50 microbial entries was developed that allowed bacterial speciation of the ESKAPE pathogens, as well as identification of pathogens directly from blood bottles without culture on solid medium and determination of antimicrobial peptide resistance. These results demonstrate that bacterial glycolipid mass spectra represent chemical barcodes that identify pathogens, potentially providing a useful alternative to existing diagnostics.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry.
- Record: found
- Abstract: found
- Article: not found
Matrix-assisted laser desorption ionization-time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology.

- Record: found
- Abstract: found
- Article: found