- Record: found
- Abstract: found
- Article: found
Polyketide synthases of Diaporthe helianthi and involvement of DhPKS1 in virulence on sunflower
Read this article at
Abstract
Background
The early phases of Diaporthe helianthi pathogenesis on sunflower are characterized by the production of phytotoxins that may play a role in host colonisation. In previous studies, phytotoxins of a polyketidic nature were isolated and purified from culture filtrates of virulent strains of D. helianthi isolated from sunflower. A highly aggressive isolate (7/96) from France contained a gene fragment of a putative nonaketide synthase ( lovB) which was conserved in a virulent D. helianthi population.
Results
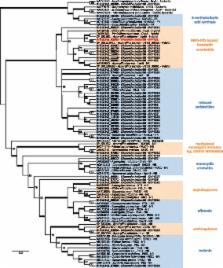
In order to investigate the role of polyketide synthases in D. helianthi 7/96, a draft genome of this isolate was examined. We were able to find and phylogenetically analyse 40 genes putatively coding for polyketide synthases (PKSs). Analysis of their domains revealed that most PKS genes of D. helianthi are reducing PKSs, whereas only eight lacked reducing domains. Most of the identified PKSs have orthologs shown to be virulence factors or genetic determinants for toxin production in other pathogenic fungi. One of the genes ( DhPKS1) corresponded to the previously cloned D. helianthi lovB gene fragment and clustered with a nonribosomal peptide synthetase (NRPS) -PKS hybrid/lovastatin nonaketide like A. nidulans LovB. We used DhPKS1 as a case study and carried out its disruption through Agrobacterium-mediated transformation in the isolate 7/96. D. helianthi DhPKS1 deleted mutants were less virulent to sunflower compared to the wild type, indicating a role for this gene in the pathogenesis of the fungus.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans.
- Record: found
- Abstract: found
- Article: not found
Agrobacterium tumefaciens-mediated transformation of filamentous fungi.
- Record: found
- Abstract: found
- Article: not found