- Record: found
- Abstract: found
- Article: found
Enteroaggregative Escherichia coli Adherence Fimbriae Drive Inflammatory Cell Recruitment via Interactions with Epithelial MUC1
Read this article at
ABSTRACT
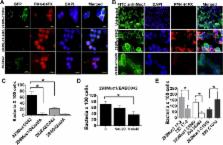
Enteroaggregative Escherichia coli (EAEC) causes diarrhea and intestinal inflammation worldwide. EAEC strains are characterized by the presence of aggregative adherence fimbriae (AAF), which play a key role in pathogenesis by mediating attachment to the intestinal mucosa and by triggering host inflammatory responses. Here, we identify the epithelial transmembrane mucin MUC1 as an intestinal host cell receptor for EAEC, demonstrating that AAF-mediated interactions between EAEC and MUC1 facilitate enhanced bacterial adhesion. We further demonstrate that EAEC infection also causes elevated expression of MUC1 in inflamed human intestinal tissues. Moreover, we find that MUC1 facilitates AAF-dependent migration of neutrophils across the epithelium in response to EAEC infection. Thus, we show for the first time a proinflammatory role for MUC1 in the host response to an intestinal pathogen.
IMPORTANCE
EAEC is a clinically important intestinal pathogen that triggers intestinal inflammation and diarrheal illness via mechanisms that are not yet fully understood. Our findings provide new insight into how EAEC triggers host inflammation and underscores the pivotal role of AAFs—the principal adhesins of EAEC—in driving EAEC-associated disease. Most importantly, our findings add a new dimension to the signaling properties of the transmembrane mucin MUC1. Mostly studied for its role in various forms of cancer, MUC1 is widely regarded as playing an anti-inflammatory role in response to infection with bacterial pathogens in various tissues. However, the role of MUC1 during intestinal infections has not been previously explored, and our results describe the first report of MUC1 as a proinflammatory factor following intestinal infection.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
German outbreak of Escherichia coli O104:H4 associated with sprouts.
- Record: found
- Abstract: not found
- Article: not found
A complementation analysis of the restriction and modification of DNA in Escherichia coli.
- Record: found
- Abstract: found
- Article: not found