- Record: found
- Abstract: found
- Article: found
Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology
Read this article at
Abstract
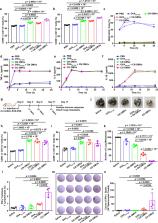
An effective tumor vaccine vector that can rapidly display neoantigens is urgently needed. Outer membrane vesicles (OMVs) can strongly activate the innate immune system and are qualified as immunoadjuvants. Here, we describe a versatile OMV-based vaccine platform to elicit a specific anti-tumor immune response via specifically presenting antigens onto OMV surface. We first display tumor antigens on the OMVs surface by fusing with ClyA protein, and then simplify the antigen display process by employing a Plug-and-Display system comprising the tag/catcher protein pairs. OMVs decorated with different protein catchers can simultaneously display multiple, distinct tumor antigens to elicit a synergistic antitumour immune response. In addition, the bioengineered OMVs loaded with different tumor antigens can abrogate lung melanoma metastasis and inhibit subcutaneous colorectal cancer growth. The ability of the bioengineered OMV-based platform to rapidly and simultaneously display antigens may facilitate the development of these agents for personalized tumour vaccines.
Abstract
Outer membrane vesicles (OMVs), non-replicative particles secreted by Gram-negative bacteria, are known for their immunostimulatory and adjuvant properties. Here, by employing a Plug-and-Display technology, the authors engineer OMVs to display tumor antigens on the surface, a platform that promotes anti-tumor immune responses in preclinical cancer models.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Oncology meets immunology: the cancer-immunity cycle.
- Record: found
- Abstract: found
- Article: not found
Neoantigens in cancer immunotherapy.
- Record: found
- Abstract: found
- Article: not found