- Record: found
- Abstract: found
- Article: found
Dual-responsive supramolecular photodynamic nanomedicine with activatable immunomodulation for enhanced antitumor therapy
Read this article at
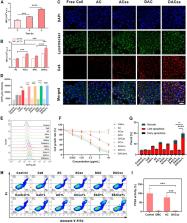
Abstract
A major challenge facing photodynamic therapy (PDT) is that the activity of the immune-induced infiltrating CD8 + T cells is subject to the regulatory T lymphocytes (Tregs), leaving the tumor at risk of recurrence and metastasis after the initial ablation. To augment the antitumor response and reprogram the immunosuppressive tumor microenvironment (TME), a supramolecular photodynamic nanoparticle (DACss) is constructed by the host-guest interaction between demethylcantharidin-conjugated β-cyclodextrin (DMC-CD) and amantadine-terminated disulfide-conjugated FFVLGGGC peptide with chlorin e6 decoration (Ad-ss-pep-Ce6) to achieve intelligent delivery of photosensitizer and immunomodulator for breast cancer treatment. The acid-labile β-carboxamide bond of DMC-CD is hydrolyzed in response to the acidic TME, resulting in the localized release of DMC and subsequent inhibition of Tregs. The guest molecule Ad-ss-pep-Ce6 can be cleaved by a high level of intracellular GSH, reducing photosensitizer toxicity and increasing photosensitizer retention in the tumor. With a significant increase in the CTL/Treg ratio, the combination of Ce6-based PDT and DMC-mediated immunomodulation adequately achieved spatiotemporal regulation and remodeling of the TME, as well as improved primary tumor and in situ lung metastasis suppression with the aid of PD-1 antibody.
Graphical abstract
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Colorectal cancer statistics, 2020
- Record: found
- Abstract: found
- Article: not found
IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade
- Record: found
- Abstract: found
- Article: not found
